Technology peripherals
Technology peripherals
 AI
AI
 New standard for AI imaging, only 1% of original data can achieve the best performance, general medical basic model published in Nature sub-journal
New standard for AI imaging, only 1% of original data can achieve the best performance, general medical basic model published in Nature sub-journal
New standard for AI imaging, only 1% of original data can achieve the best performance, general medical basic model published in Nature sub-journal

Editor | Cabbage Leaf
The large-scale pre-trained base model has achieved great success in non-medical fields. However, training these models often requires large, comprehensive datasets, in contrast to the smaller and more specialized datasets common in biomedical imaging.
Researchers at the Fraunhofer Institute for Digital Medicine MEVIS in Germany proposed a multi-task learning strategy that separates the number of training tasks from memory requirements.
They trained a universal biomedical pre-trained model (UMedPT) on a multi-task database including tomography, microscopy and X-ray images and employed various labeling strategies such as classification, segmentation and object detection. The UMedPT base model outperforms ImageNet pre-trained and previous STOA models.
In external independent validation, imaging features extracted using UMedPT were proven to set a new standard for cross-center transferability.
The study was titled "Overcoming data scarcity in biomedical imaging with a foundational multi-task model" and was published in "Nature Computational Science" on July 19, 2024.

Deep learning is gradually revolutionizing biomedical image analysis due to its ability to learn and extract useful image representations.
The general method is to pre-train the model on a large-scale natural image dataset (such as ImageNet or LAION), and then fine-tune it for specific tasks or directly use the pre-trained features. But fine-tuning requires more computing resources.
At the same time, the field of biomedical imaging requires a large amount of annotated data for effective deep learning pre-training, but such data is often scarce.
Multi-task learning (MTL) provides a solution to data scarcity by training a model to solve multiple tasks simultaneously. It leverages many small and medium-sized datasets in biomedical imaging to pre-train image representations suitable for all tasks and is suitable for data-scarce domains.
MTL has been applied to biomedical image analysis in a variety of ways, including training from multiple small and medium-sized datasets for different tasks, and using multiple label types on a single image, demonstrating that shared features can improve task performance.
In the latest research, in order to combine multiple datasets with different label types for large-scale pre-training, researchers from the MEVIS Institute introduced a multi-task training strategy and corresponding model architecture, specifically through learning Versatile representations across different modalities, diseases, and label types to address data scarcity in biomedical imaging.
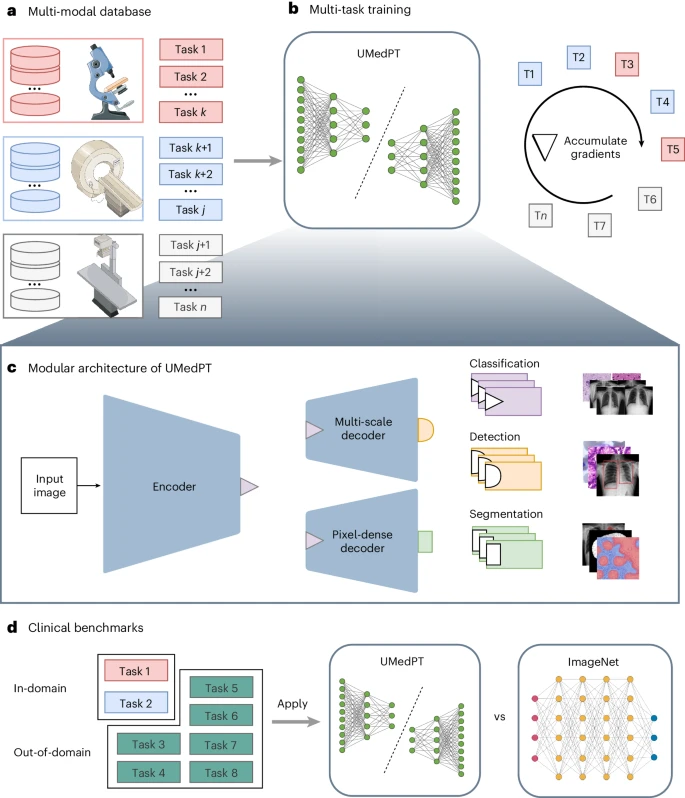
To cope with the memory constraints encountered in large-scale multi-task learning, this method adopts a gradient accumulation-based training loop, whose expansion is almost unlimited by the number of training tasks.
On this basis, the researchers trained a fully supervised biomedical imaging base model called UMedPT using 17 tasks and their original annotations.
The image below shows the architecture of the team’s neural network, which consists of shared blocks including an encoder, segmentation decoder, and localization decoder, as well as task-specific heads. Shared blocks are trained to be applicable to all pre-training tasks, helping to extract common features, while task-specific supervisors handle label-specific loss calculations and predictions.
The set tasks include three supervised label types: object detection, segmentation and classification. For example, classification tasks can model binary biomarkers, segmentation tasks can extract spatial information, and object detection tasks can be used to train biomarkers based on cell numbers.
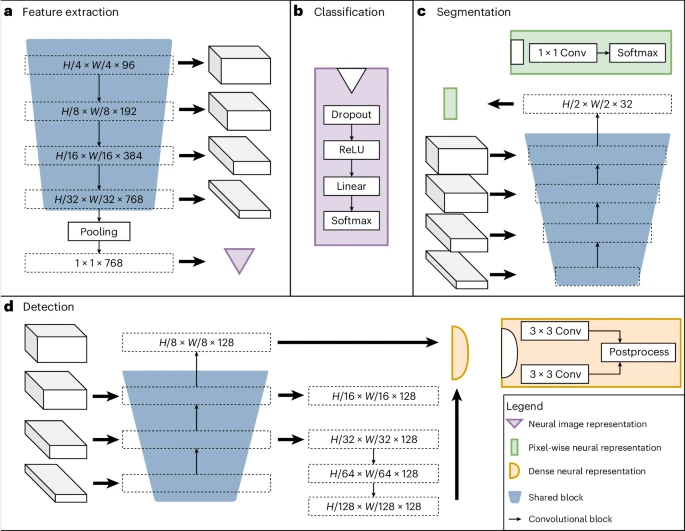
Illustration: UMedPT’s architecture. (Source: Paper)
UMedPT consistently matches or outperforms pretrained ImageNet networks on both in-domain and out-of-domain tasks, while maintaining strong performance using less training data when directly applying image representation (freezing) and fine-tuning settings.
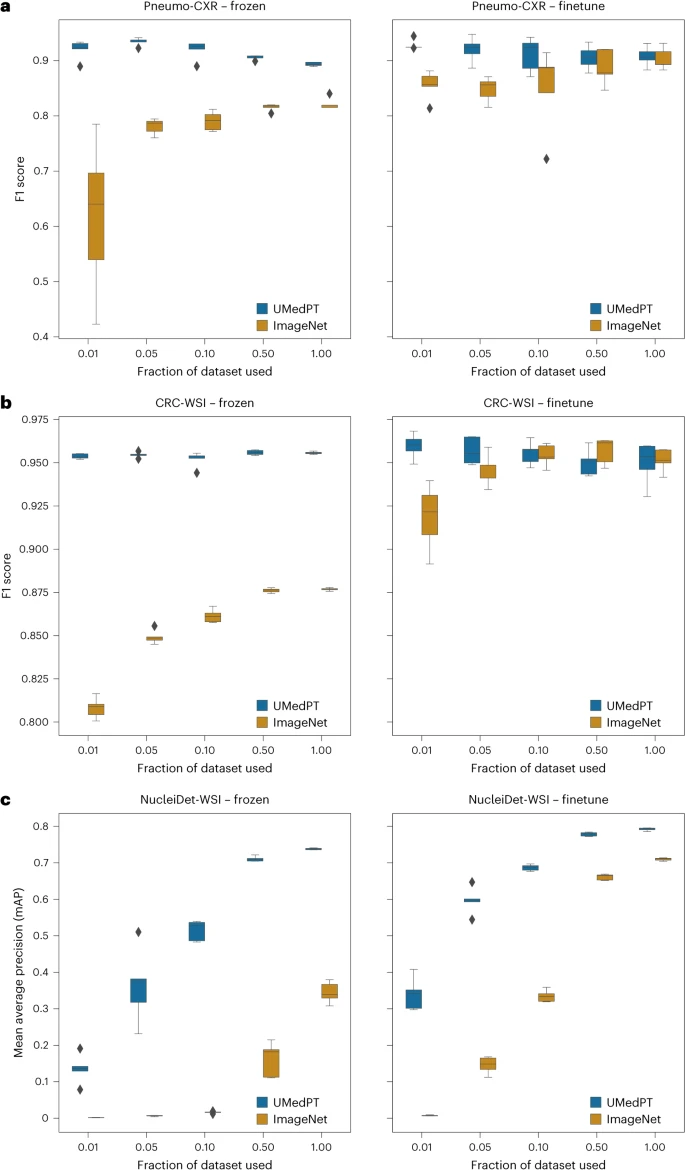
Illustration: Results of tasks within the domain. (Source: paper)
For classification tasks associated with pre-trained databases, UMedPT is able to achieve the best performance of the ImageNet baseline on all configurations using only 1% of the original training data. This model achieves higher performance using frozen encoders compared to the model using fine-tuning.
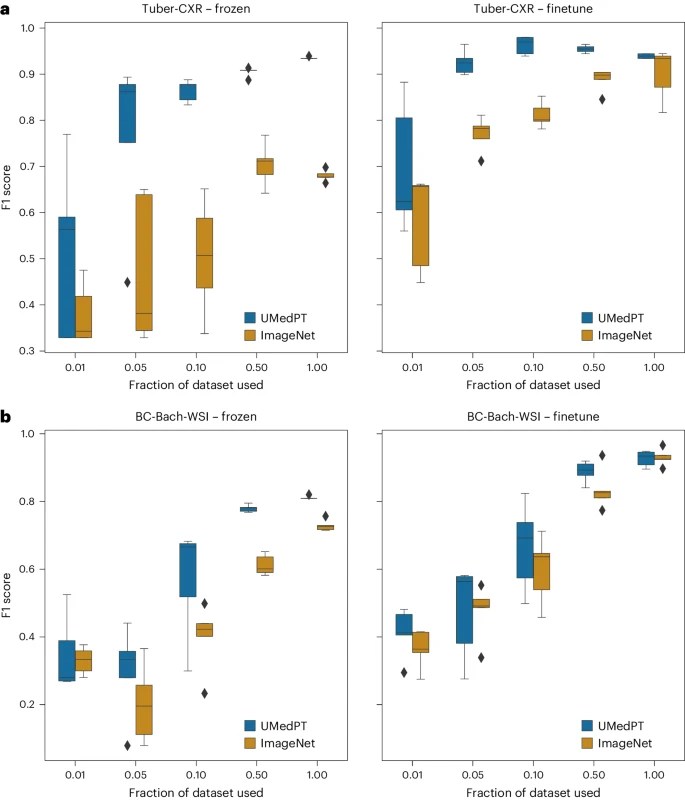
Illustration: Results for out-of-domain tasks (source: paper)
For out-of-domain tasks, UMedPT is able to match the performance of ImageNet using only 50% or less data, even with fine-tuning applied.
Additionally, the researchers compared the performance of UMedPT with results reported in the literature. When using the frozen encoder configuration, UMedPT exceeded the external reference results in most tasks. In this setting, it also outperforms the average area under the curve (AUC) in the MedMNIST database 16 .
It is worth noting that the tasks for which the frozen application of UMedPT did not outperform the reference results were outside the domain (BC-Bach-WSI for breast cancer classification and CNS-MRI for CNS tumor diagnosis). With fine-tuning, pre-training with UMedPT outperforms external reference results in all tasks.

Illustration: The amount of data required by UMedPT to achieve state-of-the-art performance on tasks in different imaging domains. (Source: Paper)
As a foundation for future developments in data-scarce fields, UMedPT opens up the prospect of deep learning applications in medical fields where collecting large amounts of data is particularly challenging, such as rare diseases and pediatric imaging.
Paper link:https://www.nature.com/articles/s43588-024-00662-z
Related content:https://www.nature.com/articles/s43588-024-00658- 9
The above is the detailed content of New standard for AI imaging, only 1% of original data can achieve the best performance, general medical basic model published in Nature sub-journal. For more information, please follow other related articles on the PHP Chinese website!

Hot AI Tools

Undresser.AI Undress
AI-powered app for creating realistic nude photos

AI Clothes Remover
Online AI tool for removing clothes from photos.

Undress AI Tool
Undress images for free

Clothoff.io
AI clothes remover

Video Face Swap
Swap faces in any video effortlessly with our completely free AI face swap tool!

Hot Article

Hot Tools

Notepad++7.3.1
Easy-to-use and free code editor

SublimeText3 Chinese version
Chinese version, very easy to use

Zend Studio 13.0.1
Powerful PHP integrated development environment

Dreamweaver CS6
Visual web development tools

SublimeText3 Mac version
God-level code editing software (SublimeText3)

Hot Topics
 1662
1662
 14
14
 1419
1419
 52
52
 1311
1311
 25
25
 1261
1261
 29
29
 1234
1234
 24
24
 Bytedance Cutting launches SVIP super membership: 499 yuan for continuous annual subscription, providing a variety of AI functions
Jun 28, 2024 am 03:51 AM
Bytedance Cutting launches SVIP super membership: 499 yuan for continuous annual subscription, providing a variety of AI functions
Jun 28, 2024 am 03:51 AM
This site reported on June 27 that Jianying is a video editing software developed by FaceMeng Technology, a subsidiary of ByteDance. It relies on the Douyin platform and basically produces short video content for users of the platform. It is compatible with iOS, Android, and Windows. , MacOS and other operating systems. Jianying officially announced the upgrade of its membership system and launched a new SVIP, which includes a variety of AI black technologies, such as intelligent translation, intelligent highlighting, intelligent packaging, digital human synthesis, etc. In terms of price, the monthly fee for clipping SVIP is 79 yuan, the annual fee is 599 yuan (note on this site: equivalent to 49.9 yuan per month), the continuous monthly subscription is 59 yuan per month, and the continuous annual subscription is 499 yuan per year (equivalent to 41.6 yuan per month) . In addition, the cut official also stated that in order to improve the user experience, those who have subscribed to the original VIP
 Breaking through the boundaries of traditional defect detection, 'Defect Spectrum' achieves ultra-high-precision and rich semantic industrial defect detection for the first time.
Jul 26, 2024 pm 05:38 PM
Breaking through the boundaries of traditional defect detection, 'Defect Spectrum' achieves ultra-high-precision and rich semantic industrial defect detection for the first time.
Jul 26, 2024 pm 05:38 PM
In modern manufacturing, accurate defect detection is not only the key to ensuring product quality, but also the core of improving production efficiency. However, existing defect detection datasets often lack the accuracy and semantic richness required for practical applications, resulting in models unable to identify specific defect categories or locations. In order to solve this problem, a top research team composed of Hong Kong University of Science and Technology Guangzhou and Simou Technology innovatively developed the "DefectSpectrum" data set, which provides detailed and semantically rich large-scale annotation of industrial defects. As shown in Table 1, compared with other industrial data sets, the "DefectSpectrum" data set provides the most defect annotations (5438 defect samples) and the most detailed defect classification (125 defect categories
 NVIDIA dialogue model ChatQA has evolved to version 2.0, with the context length mentioned at 128K
Jul 26, 2024 am 08:40 AM
NVIDIA dialogue model ChatQA has evolved to version 2.0, with the context length mentioned at 128K
Jul 26, 2024 am 08:40 AM
The open LLM community is an era when a hundred flowers bloom and compete. You can see Llama-3-70B-Instruct, QWen2-72B-Instruct, Nemotron-4-340B-Instruct, Mixtral-8x22BInstruct-v0.1 and many other excellent performers. Model. However, compared with proprietary large models represented by GPT-4-Turbo, open models still have significant gaps in many fields. In addition to general models, some open models that specialize in key areas have been developed, such as DeepSeek-Coder-V2 for programming and mathematics, and InternVL for visual-language tasks.
 Training with millions of crystal data to solve the crystallographic phase problem, the deep learning method PhAI is published in Science
Aug 08, 2024 pm 09:22 PM
Training with millions of crystal data to solve the crystallographic phase problem, the deep learning method PhAI is published in Science
Aug 08, 2024 pm 09:22 PM
Editor |KX To this day, the structural detail and precision determined by crystallography, from simple metals to large membrane proteins, are unmatched by any other method. However, the biggest challenge, the so-called phase problem, remains retrieving phase information from experimentally determined amplitudes. Researchers at the University of Copenhagen in Denmark have developed a deep learning method called PhAI to solve crystal phase problems. A deep learning neural network trained using millions of artificial crystal structures and their corresponding synthetic diffraction data can generate accurate electron density maps. The study shows that this deep learning-based ab initio structural solution method can solve the phase problem at a resolution of only 2 Angstroms, which is equivalent to only 10% to 20% of the data available at atomic resolution, while traditional ab initio Calculation
 Google AI won the IMO Mathematical Olympiad silver medal, the mathematical reasoning model AlphaProof was launched, and reinforcement learning is so back
Jul 26, 2024 pm 02:40 PM
Google AI won the IMO Mathematical Olympiad silver medal, the mathematical reasoning model AlphaProof was launched, and reinforcement learning is so back
Jul 26, 2024 pm 02:40 PM
For AI, Mathematical Olympiad is no longer a problem. On Thursday, Google DeepMind's artificial intelligence completed a feat: using AI to solve the real question of this year's International Mathematical Olympiad IMO, and it was just one step away from winning the gold medal. The IMO competition that just ended last week had six questions involving algebra, combinatorics, geometry and number theory. The hybrid AI system proposed by Google got four questions right and scored 28 points, reaching the silver medal level. Earlier this month, UCLA tenured professor Terence Tao had just promoted the AI Mathematical Olympiad (AIMO Progress Award) with a million-dollar prize. Unexpectedly, the level of AI problem solving had improved to this level before July. Do the questions simultaneously on IMO. The most difficult thing to do correctly is IMO, which has the longest history, the largest scale, and the most negative
 PRO | Why are large models based on MoE more worthy of attention?
Aug 07, 2024 pm 07:08 PM
PRO | Why are large models based on MoE more worthy of attention?
Aug 07, 2024 pm 07:08 PM
In 2023, almost every field of AI is evolving at an unprecedented speed. At the same time, AI is constantly pushing the technological boundaries of key tracks such as embodied intelligence and autonomous driving. Under the multi-modal trend, will the situation of Transformer as the mainstream architecture of AI large models be shaken? Why has exploring large models based on MoE (Mixed of Experts) architecture become a new trend in the industry? Can Large Vision Models (LVM) become a new breakthrough in general vision? ...From the 2023 PRO member newsletter of this site released in the past six months, we have selected 10 special interpretations that provide in-depth analysis of technological trends and industrial changes in the above fields to help you achieve your goals in the new year. be prepared. This interpretation comes from Week50 2023
 To provide a new scientific and complex question answering benchmark and evaluation system for large models, UNSW, Argonne, University of Chicago and other institutions jointly launched the SciQAG framework
Jul 25, 2024 am 06:42 AM
To provide a new scientific and complex question answering benchmark and evaluation system for large models, UNSW, Argonne, University of Chicago and other institutions jointly launched the SciQAG framework
Jul 25, 2024 am 06:42 AM
Editor |ScienceAI Question Answering (QA) data set plays a vital role in promoting natural language processing (NLP) research. High-quality QA data sets can not only be used to fine-tune models, but also effectively evaluate the capabilities of large language models (LLM), especially the ability to understand and reason about scientific knowledge. Although there are currently many scientific QA data sets covering medicine, chemistry, biology and other fields, these data sets still have some shortcomings. First, the data form is relatively simple, most of which are multiple-choice questions. They are easy to evaluate, but limit the model's answer selection range and cannot fully test the model's ability to answer scientific questions. In contrast, open-ended Q&A
 The accuracy rate reaches 60.8%. Zhejiang University's chemical retrosynthesis prediction model based on Transformer was published in the Nature sub-journal
Aug 06, 2024 pm 07:34 PM
The accuracy rate reaches 60.8%. Zhejiang University's chemical retrosynthesis prediction model based on Transformer was published in the Nature sub-journal
Aug 06, 2024 pm 07:34 PM
Editor | KX Retrosynthesis is a critical task in drug discovery and organic synthesis, and AI is increasingly used to speed up the process. Existing AI methods have unsatisfactory performance and limited diversity. In practice, chemical reactions often cause local molecular changes, with considerable overlap between reactants and products. Inspired by this, Hou Tingjun's team at Zhejiang University proposed to redefine single-step retrosynthetic prediction as a molecular string editing task, iteratively refining the target molecular string to generate precursor compounds. And an editing-based retrosynthetic model EditRetro is proposed, which can achieve high-quality and diverse predictions. Extensive experiments show that the model achieves excellent performance on the standard benchmark data set USPTO-50 K, with a top-1 accuracy of 60.8%.