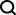kj is kilocalories or kilojoules
kj is kilojoules; kilojoules are a measure of the calories contained in food and the calories consumed per unit time of a certain exercise. Weight is controlled by controlling the calories consumed in food and the calories consumed by exercise, and kilocalories It is the amount of heat required for 1 gram of water to increase by 1°C at 1 atmosphere of pressure; the symbol J is named in honor of the British physicist Joule.

#The operating environment of this tutorial: Windows 7 system, Dell G3 computer.
kj Is it kilocalories or kilojoules?
kj is kilojoules.
Kilojoules are a measure of the calories contained in food and the calories consumed per unit time of certain types of exercise. Weight is controlled by controlling the calories consumed in food and the calories consumed by exercise. The symbol J is named in honor of the British physicist Joule. 1 joule = 1 N·m, which is also equal to the work done by 1 watt of power in 1 second, 1 joule = 1 watt·second.
Kcal is the amount of heat required for 1 gram of water to rise 1°C at 1 atmosphere. Calories are units of energy that are widely used in nutritional measurement and fitness manuals. The international standard unit of energy is joule, 1 calorie = 4.1868 joules.

Kilogram conversion formula
1 calorie is equal to 4.2 joules Calorie unit conversion: 1 kilocalorie (kcal) = 1 kcal = 4.1868 kilojoules (kJ )=1 cal/g. The relationship between
and Joule is: 1 card 20℃=4.1868J
Some countries use the card 15℃. The relationship with Joules is: 1 cal 15°C = 4.1855J
Some countries use the international steam table card IT. The relationship with Joule is: 1 card IT=4.1868J
For more related knowledge, please visit the FAQ column!
The above is the detailed content of kj is kilocalories or kilojoules. For more information, please follow other related articles on the PHP Chinese website!

Hot AI Tools

Undresser.AI Undress
AI-powered app for creating realistic nude photos

AI Clothes Remover
Online AI tool for removing clothes from photos.

Undress AI Tool
Undress images for free

Clothoff.io
AI clothes remover

AI Hentai Generator
Generate AI Hentai for free.

Hot Article

Hot Tools

Notepad++7.3.1
Easy-to-use and free code editor

SublimeText3 Chinese version
Chinese version, very easy to use

Zend Studio 13.0.1
Powerful PHP integrated development environment

Dreamweaver CS6
Visual web development tools

SublimeText3 Mac version
God-level code editing software (SublimeText3)

Hot Topics
 1378
1378
 52
52