Technology peripherals
Technology peripherals
 AI
AI
 Understand the whole picture of AI pharmaceuticals in one article: annual revenue of 30 billion, with three distinct echelons
Understand the whole picture of AI pharmaceuticals in one article: annual revenue of 30 billion, with three distinct echelons
Understand the whole picture of AI pharmaceuticals in one article: annual revenue of 30 billion, with three distinct echelons
How popular are AI pharmaceuticals that have been raising rounds of financing?
A foreign order has reached a maximum of 33.1 billion yuan, which is close to the R&D investment of a traditional pharmaceutical company for a whole year.
The domestic market is conservatively estimated to reach 204 billion yuan. Internet leading companies such as BAT, Byte, Huawei, etc. are vying for investment. Some companies have even completed three rounds of large-scale financing within a year...
△Picture source: Qubit Think Tank
From Peking University’s Frontier Interdisciplinary Research Institute, professors from famous foreign universities, to MIT PhDs, they have joined in entrepreneurship, and some have even received foreign awards. Students with Ph.D. offers from prestigious universities dropped out to join...
However, in sharp contrast to the capital boom, is the current situation of industry development:
The number of domestic AI pharmaceutical companies listed today is 0 , not even one has achieved profitability yet; the stock prices of foreign AI pharmaceutical companies have plummeted wildly after listing.
Currently, no drug successfully developed by AI has been successfully launched in the world. According to public information from various start-up companies, there are only 2 domestic companies and 8 foreign companies whose pipelines have just entered the clinical stage. period stage.
Now that the initial enthusiasm in the industry has passed, voices of doubt have become increasingly prominent:
Is the AI pharmaceutical industry a star track that attracts attention in future investment and financing, or is it a PPT bubble under the disguise of technology?
Can the data bottleneck of AI technology itself and its role in the pharmaceutical field really save the declining profits of traditional pharmaceutical companies?
When will AI pharmaceuticals really come to fruition?
After interviewing dozens of institutions, we wrote the "AI Pharmaceutical In-Depth Industry Report", trying to describe the current situation of the AI pharmaceutical industry at home and abroad, as well as the difficulties and opportunities faced by this industry.
AI Pharmaceutical "Current Situation Map"
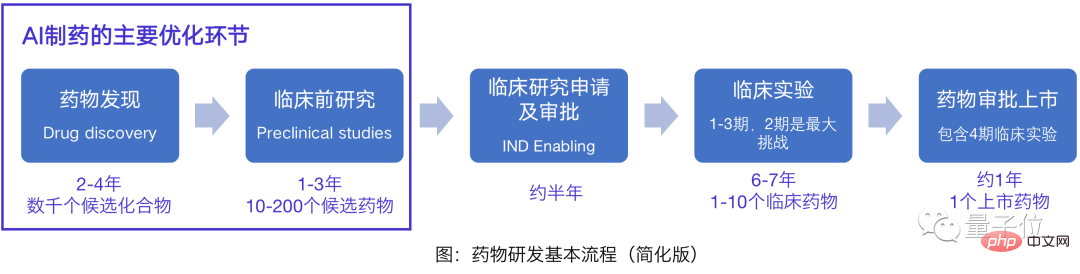
AI Pharmaceutical, more accurately, should be "using AI to predict drugs."
Yes, AI at this stage has not really broken the traditional pharmaceutical research and development system. Even from the perspective of the research and development process, AI optimization is less than 40%.

Such positioning intensifies the "ambivalence" of AI pharmaceuticals:
On the one hand, drug discovery is the cornerstone of the entire drug research and development process , is also the most promising breakthrough for drug innovation; on the other hand, 60-80% of the clinical trial costs of drug research and development cannot be optimized by AI.
This sense of contradiction is also reflected in the financing situation, technology pricing and R&D implementation of AI pharmaceuticals.
Just looking at the financing situation, one would think that AI pharmaceuticals is an industry with a lot of money prospects.
According to data from Bank of China Securities, in 2020 alone, the number of AI pharmaceutical financing projects in China doubled, and the total financing in the same year increased by about 10 times year-on-year.
Since then, at least 11 AI pharmaceutical companies around the world have received large financing of more than US$100 million. And this data is still showing a rising trend:
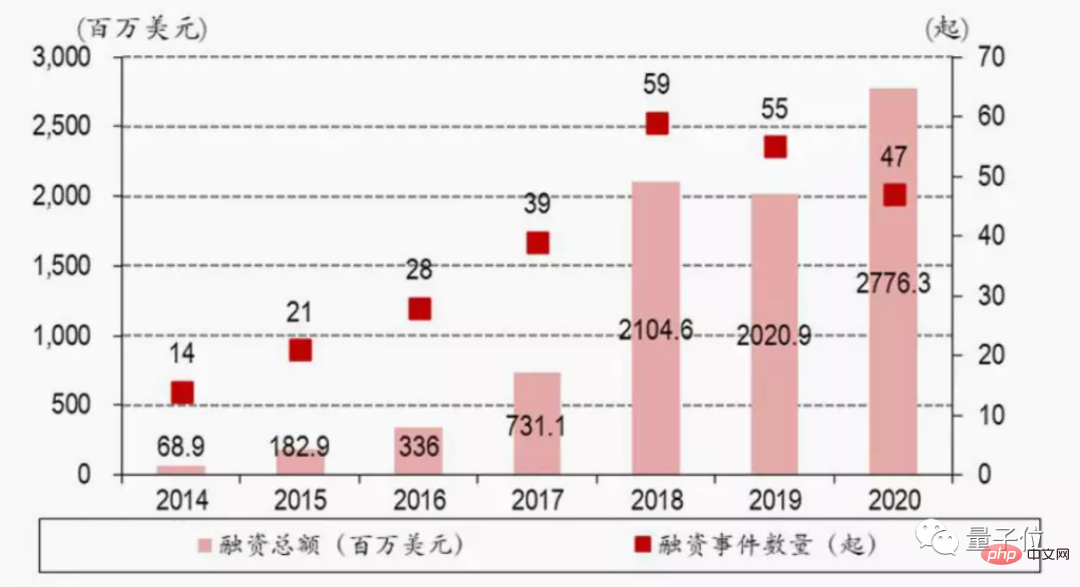
△Data source: BOC Securities
According to Arterial Orange The report shows that "AI pharmaceuticals" has become one of the most popular tracks for capital in 2021, with 77 global financings totaling US$4.56 billion (approximately RMB 30.7 billion), of which US$1.24 billion was raised in the Chinese market.
At the same time, the survival situation of AI pharmaceutical companies is also very optimistic. About 53% of Series A companies entered Series B; 38% of Series B companies successfully entered Series C; 46% of Series C companies successfully entered Series D.
Looking at the monetization methods of AI companies, it seems that they also have investment potential.
Referring to the Benevolent prospectus data, looking at the price of the self-developed pipeline alone, the down payment and milestone payment prices set by AI pharmaceutical companies are not low, especially after the second phase of clinical trials, the down payment alone can reach nearly 1 One hundred million U.S. dollars.

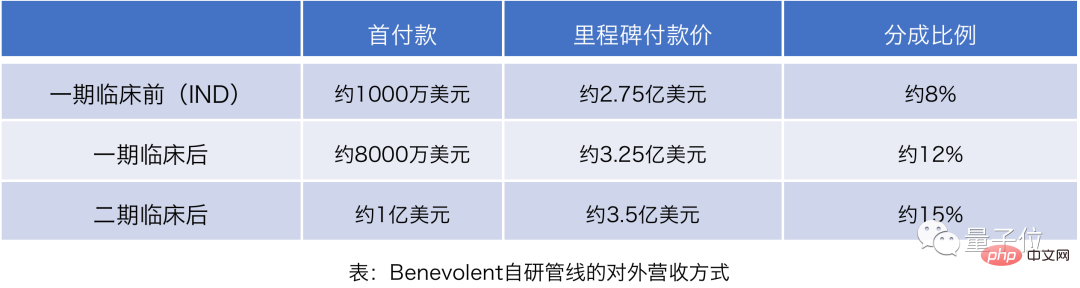
△Picture source: Quantum Think Tank
However, combined with the research and development implementation situation, there is a strong sense of contradiction.
For example, there is still no AI-predicted drug on the market in the industry, and there is not even any drug on the market that has publicly entered the second phase of clinical trials.
At the same time, AI pharmaceuticals has yet to emerge with a breakthrough core technology that can prove that AI for drug discovery (AIDD) is reliable and sustainable and can replace or optimize the traditional computer drug discovery (CADD) process. .
According to data from the Qubit Think Tank, even the fastest-growing AI prediction drugs have only passed animal testing and entered the first phase of clinical trials.
Among these fastest-growing AI prediction drugs, there are only 3 domestic pipelines. Although nearly 16 foreign pipelines have entered clinical trials, they are all still in the first phase.
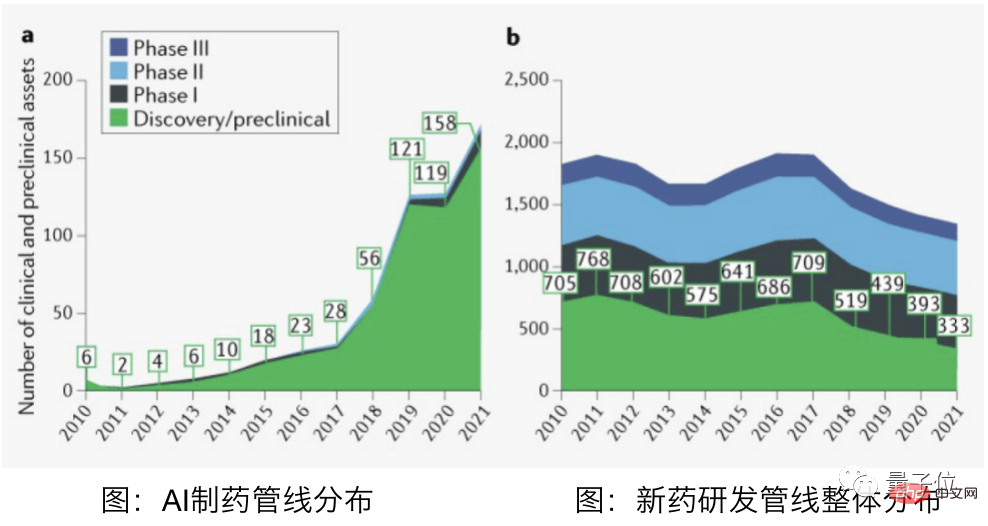

△Picture source: Qubit Think Tank
This situation has led to a gradual cooling off of capital enthusiasm since 2021:
Currently, no domestic AI pharmaceutical company has completed its listing, and no company has achieved profitability.
The stock prices of at least 7 or 8 foreign listed companies have almost without exception plummeted.
In fact, judging from past experience, the probability of drug research and development failure is extremely high. The investment in clinical trials of countless new drugs has ultimately been wasted, which once again increases the uncertainty of AI prediction of drug launch. sex.
Especially since these drugs have not yet entered the second phase of clinical trials, there is no guarantee whether they can be launched on the market.
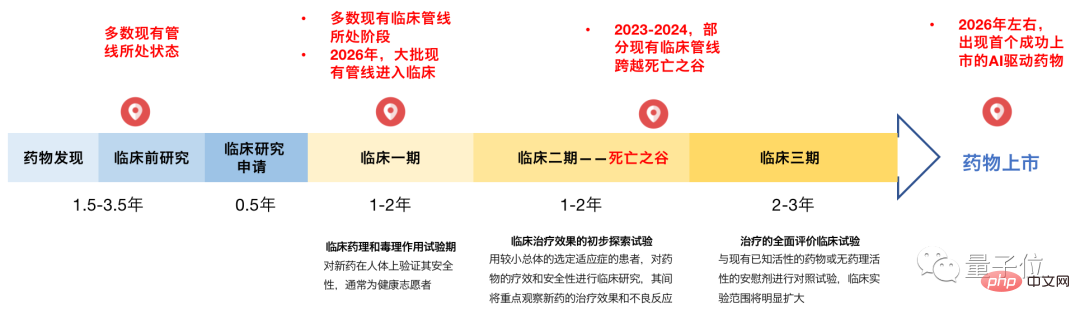
Since the "Thalidomide Incident" in 1961, whether the effectiveness of a drug can be verified has always been the biggest threshold for all new drugs to be launched. If "substantial evidence" such as credible safety data and clear benefit data for patients cannot be provided, the drug is very likely to die at this stage.
Obviously, during this period, most capital will be on the sidelines until drugs predicted by AI enter and pass Phase II clinical trials.
Compared with traditional pharmaceutical companies, AI pharmaceutical companies are not large in size. In situations where the risks and costs of clinical trials are extremely high, this “possibility of failure” is either transferred or can only be borne by the company itself.
With this, AI pharmaceutical companies have formed two main business models.
The first is the CRO (Contract Research Organization) model that transfers R&D risks. The company will "outsource" to traditional pharmaceutical companies or other companies and use AI technology to predict the drugs that Party A needs.
The second is the self-development pipeline model that is willing to bear the risk of R&D failure. The company holds the drug and technology patents in its hands. Once it is successfully launched or reaches a specific realization node (such as pre-clinical research), it can Make money by transferring patents or charging fees.
How to decide whether to be a CRO or self-developed pipeline?
One is the funding situation. The funds required for self-research pipelines are extremely high. Companies that are not short of money can directly develop their own pipelines; companies that want to develop their own pipelines but lack funds can first make money through CRO outsourcing, and then use the money earned to develop their own pipelines.
The other is the positioning difference. Compared with the large number of pharmaceutical-related theories mastered by companies originating from traditional pharmaceutical companies, CROs are more suitable for "cross-border entrepreneurial" players to quickly establish their own AI technology signature; self-research pipelines require higher pharmaceutical experience and resources.
In fact, CRO is currently more popular in China. In comparison, it makes profits faster, has a clearer monetization model, and does not need to bear the risk costs of subsequent clinical trials.
In addition, companies that have no interest in holding AI drug patents and only sell technical services can also only be CROs.
This has also led to the third business model - those who specialize in technology platforms and sell AI pharmaceutical software to other companies for predictive research and development. However, currently there are very few domestic companies that actually charge fees.
Obviously, the positioning and technological advantages of AI pharmaceutical companies will largely affect their choice of business model.
There are many players who have entered the game, ranging from doctoral professors from prestigious domestic and foreign universities, to Internet giants and traditional pharmaceutical companies, to capital incubation, showing a diversified trend.
The first is the situation of doctoral professors from famous universities starting their own businesses. Taking Jingtai Technology as an example, it is a typical case of an MIT PhD in quantum physics returning to China to start a business. Since the company's advantage lies in AI technology and its ability to lead the industry with the help of theoretical research in quantum physics, Jingtai Technology has made it clear that it will focus on the CRO model and not develop its own pipeline.
There are also cases of university professors transforming research results. For example, Huashen Intellectual Medicine was founded by UIUC tenured professor Peng Jian. Previously, there have been relevant achievements in the field of protein molecular prediction. This venture will focus on the development of technology platforms. Build and deliver.
After this, Internet giants and traditional pharmaceutical companies have also entered the game.
The former has its own advantages in algorithmic computing power, and it is easy to use the influence of the Internet itself to quickly expand its "sphere of influence." For example, Baidu and Tencent have established Baitu Biotech and Yunshen Pharmaceutical platforms to quickly enter the industry using their accumulated AI algorithm experience. Alibaba quickly established upstream and downstream relationships by virtue of its computing power advantage.

The latter has profound drug research and development experience, and has established an AI pharmaceutical research and development team on this basis. For example, AstraZeneca, Merck, Pfizer and Teva have cooperated with Amazon AION Labs was established jointly with the Israel Biofund.
Finally, there are capital entrepreneurship and fund incubation situations. The cash flow support is sufficient, and even investors themselves have transformed into AI entrepreneurship. For example, Wang Yikai, the founder of Coin Biotech, was the vice president of Fengrui Capital. After establishing the company, It received investment from Fengrui Capital.
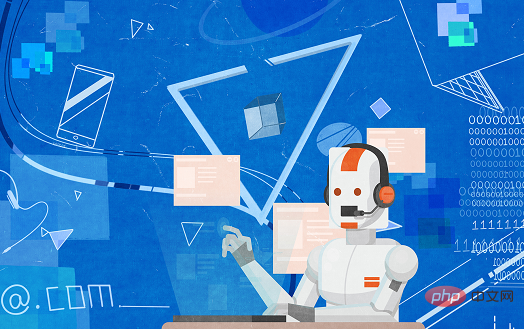
According to data from the Qubit Think Tank, the AI pharmaceutical market is expected to reach 7.2 billion in 2025 and 204 billion in 2035.
Suddenly, many players poured into the AI pharmaceutical track. However, judging from the current situation and the current situation of players, it is impossible to judge the development prospects of AI pharmaceutical companies through pure technical strength or financial advantages.
Who is the real player among them and the company most promising to take the lead in launching the first new AI drug?
Who can be the first to market the first AI drug?
There are many standards and dimensions, but there are 4 core dimensions that cannot be bypassed in the industry:
01, number of pipelines and R&D progress
Given the complexity of the pharmaceutical process, the failure rate is high , it is an extremely long process from clinical approval, research to final marketing. For the current stage, the number of pipelines is one of the most direct manifestations of strength.
As mentioned earlier, the pipeline is divided into self-development pipeline and external cooperation pipeline (CRO).
For self-developed pipelines, companies can transfer pipeline results at specific nodes, such as new targets, drug candidates, etc.; they can also use CRO to advance to the clinical stage. Once the research and development is successful and a marketing patent is obtained, profits will increase. Very impressive. However, the risks of self-developed pipelines are also obvious: the payment method is unclear, and there will be competition with other companies on the same pipeline.
Therefore, when paying attention to the self-development pipeline of AI pharmaceutical companies, it is necessary to pay more attention to its research and development progress and the potential of the selected drug direction.

In contrast, the number of cooperation pipelines under the CRO model is a more direct way to judge a company's technical strength. CRO refers to an AI pharmaceutical company completing a specific task of a traditional pharmaceutical company. After the down payment, the cooperation price is determined based on the progress of the task (such as drug discovery-synthesis-completion of clinical research), also known as milestone payment.
According to estimates from the Qubit think tank, the average down payment for domestic pipelines is US$2.8 million. Milestone prices fluctuate greatly depending on the specific drug, and can even reach tens of billions of yuan upon completion. Whoever gets more cooperation pipelines means that whose technical strength is more recognized by pharmaceutical companies, and more funds will be invested in research and development, entering a virtuous cycle.
Refer to a foreign cooperation between Exscientia and Sanofi in early 2022, with an initial payment of US$100 million. After completing the task, they will receive a "sky-high price contract" of US$5.2 billion, equivalent to approximately 33.1 billion yuan.
According to data from the Qubit Think Tank, a batch of AI predictive drugs entering the second phase of clinical trials will appear in 2023-2024, and the first successfully launched AI drug will appear around 2026 at the earliest.
Before the drug is launched, the number of cooperative pipelines and the research and development progress of self-developed pipelines are one of the directions for judging the technical strength of AI pharmaceutical companies.
02. Stable and reliable data sources
For the AI pharmaceutical industry, in addition to money, the most lacking thing is probably data. Traditional pharmaceutical companies are generally unwilling to use research and development as one of their core assets. Data set outflow.
But according to Qubit Think Tank, data is currently not a problem for leading AI pharmaceutical companies, and it can even achieve more competitive performance in the industry.
Therefore, how to obtain stable and reliable data is also an important criterion for judging the competitiveness of AI pharmaceutical companies.
Generally speaking, there are the following four methods to obtain AI data, and their stability and reliability have gradually improved:
(1) Public/third-party data sets
This type of data is of great significance to the current AI pharmaceutical industry, but it does not have long-term benefits and cannot help companies gain core competitiveness. Moreover, the more available data on existing targets means more complete exploration and less development value.
(2) Virtual data
This data acquisition method is through physical modeling and training data generated by AI. It is usually based on older targets such as penicillin to produce data in the short term. It doesn't seem to be of much value. It mainly provides training data for the prediction model to improve the prediction accuracy.
(3) Independent collection/foreign cooperation data
For companies with clear self-development pipelines/basic positioning, they can collect relevant data through independently building teams, or reach data cooperation relationships with pharmaceutical companies.
Abroad, Tempus, founded in 2015, builds its own tumor genome by providing cost-effective gene sequencing, data structuring, pathological image analysis and biological modeling services to hospitals, oncologists, cancer centers, etc. clinical database.
It took 4 years to build one of the largest cancer databases in the world, with nearly 1/3 of the US cancer database.
my country's Janssen Pharmaceutical Factory reached a cooperation with Tempus in 2020 and publicly stated that the main driving force of the cooperation is not algorithms but data.
(4) Independently produce experimental data through smart laboratories
This method mainly refers to directly conducting wet experiments to independently generate data in addition to dry experiments conducted in the laboratory, forming Wet and dry closed loop.
Compared with traditional wet data acquisition speed, the data acquisition speed can be greatly improved by using related technologies such as high-throughput, intelligence, automation, controllability, and CV identification of cell morphology.
In biology, dry experiments are conducted through computer simulation and bioinformatics methods. Wet experiments are conducted in the laboratory using molecular, cellular, and physiological testing methods.
Dry and wet combined experiments can help AI pharmaceutical startups create new competitive barriers in terms of data. This recognition has reached consensus in the industry.
In addition to a cross-team of biological talents and computer talents, the establishment of such a platform also requires strong hardware support, including experimental equipment and computing resources, as well as the ability to integrate these two resources.
Currently, domestic leading AI pharmaceutical companies, including Baidu Shengtu, Jingtai Technology and Yingsi Intelligent, all have such experimental platforms.
With sufficient funds, leading foreign AI pharmaceutical companies have begun to directly acquire upstream companies with exclusive data and technology.
For example, Schrödinger acquired XTAL BioStructures to expand its structural biology capabilities, and Relay Therapeutics acquired ZebiAI to gain its machine learning capabilities and large databases.
Therefore, as analyzed by Qubit Think Tank, traditional pharmaceutical companies as a whole have advantages in data, but it does not come from the data accumulated in the past, but from the complete experimental platform they have. For AI pharmaceutical startups with sufficient funds, this barrier is not high and they can quickly update to the same level.
03. Recognition of cooperative pharmaceutical companies
With the establishment of intelligent teams in traditional pharmaceutical companies, algorithms may not be able to become the long-term competitive advantage of AI pharmaceutical companies.
As mentioned earlier, AI pharmaceuticals have not broken the R&D process of the traditional pharmaceutical industry. In addition to building their own laboratory platforms and "working on it", the cooperation between AI pharmaceutical companies and pharmaceutical companies is equally important.
Therefore, the number of cooperative pharmaceutical companies and the industry status of these pharmaceutical companies have also become an intuitive evaluation criterion.
At present, leading AI pharmaceutical startups are gradually showing a monopoly in cooperation with traditional pharmaceutical companies. Overseas, taking Exscientia as an example, it has disclosed cooperation with top pharmaceutical companies including Roche, Bayer, Sanofi, GSK, Sumitomo of Japan, and Evotec.


Of course, the cooperation between traditional pharmaceutical companies and AI pharmaceutical companies is two-way: pharmaceutical companies provide databases and professional knowledge, and in turn require AI pharmaceutical companies Provide technology.


# Therefore, business cooperation with traditional pharmaceutical companies has become one of the most commonly adopted models by AI pharmaceutical companies.
According to Deep Pharma Intelligence, as of 2020, 93% of the 44 world's leading traditional pharmaceutical companies have completed cooperation arrangements. Especially among the world's top 10 pharmaceutical companies such as Roche, Novartis, and Pfizer, they have cooperated with AI pharmaceutical companies more than 6 times on average.
In addition to the status and quantitative recognition of the cooperative pharmaceutical companies, the CRO company selected by the AI pharmaceutical company is also one of the reference sources.
In the traditional pharmaceutical industry, CRO has a special status, and this feature will continue in the AI pharmaceutical industry.
AI pharmaceutical companies can become CROs of traditional pharmaceutical companies, but on the other hand, AI pharmaceutical companies also need their own CROs, including data partners, suppliers for assays and experiments, etc., to complete the application Approval, data collection, clinical trials and other tasks.
For AI pharmaceutical companies, the choice of CRO will greatly affect their clinical projects and commercialization process.
04. Break through the single characteristic of "AI improves efficiency"
As we all know, one of the current application scenarios of AI pharmaceuticals is to improve the efficiency of compound screening, but this is often done based on existing target and compound databases. of.
However, with the establishment of internal AI teams in pharmaceutical companies, the entry barriers for new AI pharmaceutical startups are continuing to rise. In addition, the current overlap in the entire industry is relatively high, and most companies’ pipelines are already Develop based on proven targets.
In other words, using AI to improve the efficiency of drug discovery is nothing new in this industry. At present, leading AI pharmaceutical companies have developed the innovative ability to use AI to explore the "pharmaceutical no man's land".
Therefore, it is very important for new start-ups to have their own unique entry point in business scenarios or technologies.
This may require AI pharmaceutical companies to start from the underlying theory, including redefining medical problems, creatively using multi-disciplinary perspectives such as physics and chemistry, redefining scenarios and problems in drug research and development, and using multiple principles to compensate for AI The errors and uncertainties inherent in the model and improve its efficiency.
Finally, under these four judging criteria, which players can take the lead?
According to the global AI pharmaceutical landscape map of the Qubit Think Tank, although most of the current leading players are foreign companies, domestic players such as Jingtai Technology and Yingsi Intelligent can also be seen:
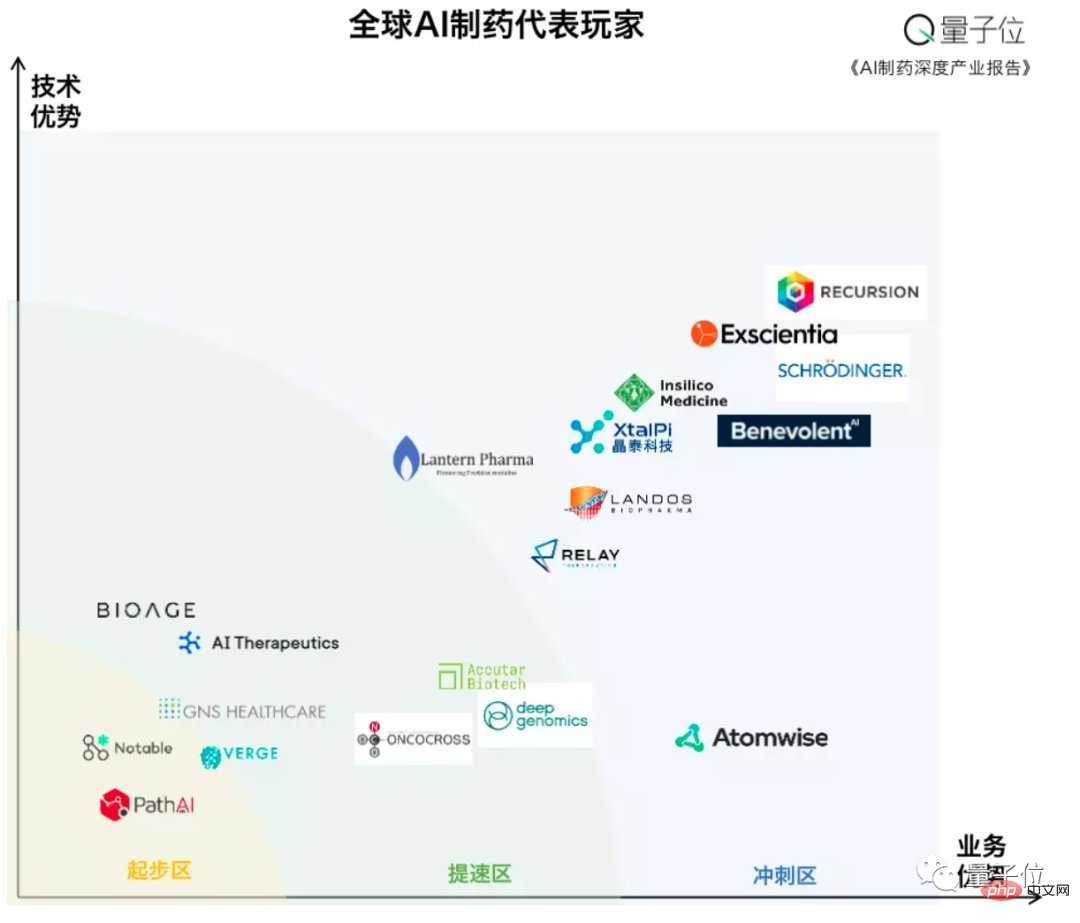
After the AI pharmaceutical track became popular, many PhD professors from famous foreign universities returned to China to start their own businesses with projects and theories, and they were also quickly making up for the lack of technological innovation capabilities in the domestic pharmaceutical industry.

This time, in the wave of pharmaceutical innovation driven by new technologies, will China give birth to a world-class pharmaceutical factory?
There is a trend and more potential.
The above is the detailed content of Understand the whole picture of AI pharmaceuticals in one article: annual revenue of 30 billion, with three distinct echelons. For more information, please follow other related articles on the PHP Chinese website!

Hot AI Tools
Undresser.AI Undress
AI-powered app for creating realistic nude photos
AI Clothes Remover
Online AI tool for removing clothes from photos.

Undress AI Tool
Undress images for free

Clothoff.io
AI clothes remover

AI Hentai Generator
Generate AI Hentai for free.

Hot Article

Hot Tools

Notepad++7.3.1
Easy-to-use and free code editor

SublimeText3 Chinese version
Chinese version, very easy to use

Zend Studio 13.0.1
Powerful PHP integrated development environment

Dreamweaver CS6
Visual web development tools

SublimeText3 Mac version
God-level code editing software (SublimeText3)

Hot Topics
 1382
1382
 52
52
 Bytedance Cutting launches SVIP super membership: 499 yuan for continuous annual subscription, providing a variety of AI functions
Jun 28, 2024 am 03:51 AM
Bytedance Cutting launches SVIP super membership: 499 yuan for continuous annual subscription, providing a variety of AI functions
Jun 28, 2024 am 03:51 AM
This site reported on June 27 that Jianying is a video editing software developed by FaceMeng Technology, a subsidiary of ByteDance. It relies on the Douyin platform and basically produces short video content for users of the platform. It is compatible with iOS, Android, and Windows. , MacOS and other operating systems. Jianying officially announced the upgrade of its membership system and launched a new SVIP, which includes a variety of AI black technologies, such as intelligent translation, intelligent highlighting, intelligent packaging, digital human synthesis, etc. In terms of price, the monthly fee for clipping SVIP is 79 yuan, the annual fee is 599 yuan (note on this site: equivalent to 49.9 yuan per month), the continuous monthly subscription is 59 yuan per month, and the continuous annual subscription is 499 yuan per year (equivalent to 41.6 yuan per month) . In addition, the cut official also stated that in order to improve the user experience, those who have subscribed to the original VIP
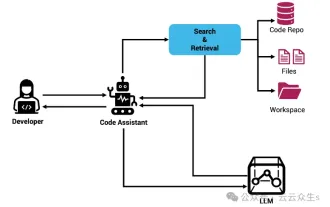 Context-augmented AI coding assistant using Rag and Sem-Rag
Jun 10, 2024 am 11:08 AM
Context-augmented AI coding assistant using Rag and Sem-Rag
Jun 10, 2024 am 11:08 AM
Improve developer productivity, efficiency, and accuracy by incorporating retrieval-enhanced generation and semantic memory into AI coding assistants. Translated from EnhancingAICodingAssistantswithContextUsingRAGandSEM-RAG, author JanakiramMSV. While basic AI programming assistants are naturally helpful, they often fail to provide the most relevant and correct code suggestions because they rely on a general understanding of the software language and the most common patterns of writing software. The code generated by these coding assistants is suitable for solving the problems they are responsible for solving, but often does not conform to the coding standards, conventions and styles of the individual teams. This often results in suggestions that need to be modified or refined in order for the code to be accepted into the application
 Can fine-tuning really allow LLM to learn new things: introducing new knowledge may make the model produce more hallucinations
Jun 11, 2024 pm 03:57 PM
Can fine-tuning really allow LLM to learn new things: introducing new knowledge may make the model produce more hallucinations
Jun 11, 2024 pm 03:57 PM
Large Language Models (LLMs) are trained on huge text databases, where they acquire large amounts of real-world knowledge. This knowledge is embedded into their parameters and can then be used when needed. The knowledge of these models is "reified" at the end of training. At the end of pre-training, the model actually stops learning. Align or fine-tune the model to learn how to leverage this knowledge and respond more naturally to user questions. But sometimes model knowledge is not enough, and although the model can access external content through RAG, it is considered beneficial to adapt the model to new domains through fine-tuning. This fine-tuning is performed using input from human annotators or other LLM creations, where the model encounters additional real-world knowledge and integrates it
 Seven Cool GenAI & LLM Technical Interview Questions
Jun 07, 2024 am 10:06 AM
Seven Cool GenAI & LLM Technical Interview Questions
Jun 07, 2024 am 10:06 AM
To learn more about AIGC, please visit: 51CTOAI.x Community https://www.51cto.com/aigc/Translator|Jingyan Reviewer|Chonglou is different from the traditional question bank that can be seen everywhere on the Internet. These questions It requires thinking outside the box. Large Language Models (LLMs) are increasingly important in the fields of data science, generative artificial intelligence (GenAI), and artificial intelligence. These complex algorithms enhance human skills and drive efficiency and innovation in many industries, becoming the key for companies to remain competitive. LLM has a wide range of applications. It can be used in fields such as natural language processing, text generation, speech recognition and recommendation systems. By learning from large amounts of data, LLM is able to generate text
 Five schools of machine learning you don't know about
Jun 05, 2024 pm 08:51 PM
Five schools of machine learning you don't know about
Jun 05, 2024 pm 08:51 PM
Machine learning is an important branch of artificial intelligence that gives computers the ability to learn from data and improve their capabilities without being explicitly programmed. Machine learning has a wide range of applications in various fields, from image recognition and natural language processing to recommendation systems and fraud detection, and it is changing the way we live. There are many different methods and theories in the field of machine learning, among which the five most influential methods are called the "Five Schools of Machine Learning". The five major schools are the symbolic school, the connectionist school, the evolutionary school, the Bayesian school and the analogy school. 1. Symbolism, also known as symbolism, emphasizes the use of symbols for logical reasoning and expression of knowledge. This school of thought believes that learning is a process of reverse deduction, through existing
 To provide a new scientific and complex question answering benchmark and evaluation system for large models, UNSW, Argonne, University of Chicago and other institutions jointly launched the SciQAG framework
Jul 25, 2024 am 06:42 AM
To provide a new scientific and complex question answering benchmark and evaluation system for large models, UNSW, Argonne, University of Chicago and other institutions jointly launched the SciQAG framework
Jul 25, 2024 am 06:42 AM
Editor |ScienceAI Question Answering (QA) data set plays a vital role in promoting natural language processing (NLP) research. High-quality QA data sets can not only be used to fine-tune models, but also effectively evaluate the capabilities of large language models (LLM), especially the ability to understand and reason about scientific knowledge. Although there are currently many scientific QA data sets covering medicine, chemistry, biology and other fields, these data sets still have some shortcomings. First, the data form is relatively simple, most of which are multiple-choice questions. They are easy to evaluate, but limit the model's answer selection range and cannot fully test the model's ability to answer scientific questions. In contrast, open-ended Q&A
 SOTA performance, Xiamen multi-modal protein-ligand affinity prediction AI method, combines molecular surface information for the first time
Jul 17, 2024 pm 06:37 PM
SOTA performance, Xiamen multi-modal protein-ligand affinity prediction AI method, combines molecular surface information for the first time
Jul 17, 2024 pm 06:37 PM
Editor | KX In the field of drug research and development, accurately and effectively predicting the binding affinity of proteins and ligands is crucial for drug screening and optimization. However, current studies do not take into account the important role of molecular surface information in protein-ligand interactions. Based on this, researchers from Xiamen University proposed a novel multi-modal feature extraction (MFE) framework, which for the first time combines information on protein surface, 3D structure and sequence, and uses a cross-attention mechanism to compare different modalities. feature alignment. Experimental results demonstrate that this method achieves state-of-the-art performance in predicting protein-ligand binding affinities. Furthermore, ablation studies demonstrate the effectiveness and necessity of protein surface information and multimodal feature alignment within this framework. Related research begins with "S
 SK Hynix will display new AI-related products on August 6: 12-layer HBM3E, 321-high NAND, etc.
Aug 01, 2024 pm 09:40 PM
SK Hynix will display new AI-related products on August 6: 12-layer HBM3E, 321-high NAND, etc.
Aug 01, 2024 pm 09:40 PM
According to news from this site on August 1, SK Hynix released a blog post today (August 1), announcing that it will attend the Global Semiconductor Memory Summit FMS2024 to be held in Santa Clara, California, USA from August 6 to 8, showcasing many new technologies. generation product. Introduction to the Future Memory and Storage Summit (FutureMemoryandStorage), formerly the Flash Memory Summit (FlashMemorySummit) mainly for NAND suppliers, in the context of increasing attention to artificial intelligence technology, this year was renamed the Future Memory and Storage Summit (FutureMemoryandStorage) to invite DRAM and storage vendors and many more players. New product SK hynix launched last year