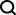Technology peripherals
Technology peripherals
 AI
AI
 Shi Yigong and other teams appear on the cover of Science: AI and cryo-electron microscopy reveal the 'atomic level' NPC structure, a breakthrough in life sciences
Shi Yigong and other teams appear on the cover of Science: AI and cryo-electron microscopy reveal the 'atomic level' NPC structure, a breakthrough in life sciences
Shi Yigong and other teams appear on the cover of Science: AI and cryo-electron microscopy reveal the 'atomic level' NPC structure, a breakthrough in life sciences
Before starting the text, let’s take a look at a picture. In the picture below, it is obvious that the right half of the picture represents richer information and a clearer structure. The left half of the picture in 2016 has a relatively simple structure and represents less information:
In fact, what is shown above is an image of the nuclear pore complex (NPC). The nuclear pore complex, composed of approximately 1,000 protein subunits, is responsible for the busy transport of macromolecules between the nucleus and cytoplasm of eukaryotic cells. It is also its only two-way channel connecting the cytoplasm and nucleus. In addition to coordinating transport, NPCs organize essential life events such as transcription, mRNA maturation, spliceosome and ribosome assembly. The powerful role of NPC has become a key point in disease mutation and host-pathogen interactions.
Thanks to the development of low-resolution whole nuclear pore structure and high-resolution nuclear pore composition structure technology, cell nuclear pores have received more and more attention. However, using this information to correctly assemble more than 30 different protein copies and construct high-resolution three-dimensional structures has been a difficult challenge.
Today, "Science" magazine published 5 papers as cover features, 3 of which jointly revealed the near-atomic resolution cryo-electron microscopy structure of the human nuclear pore complex. Two other studies presented single-particle cryo-EM images of the vertebrate nuclear pore complex in Xenopus laevis. This cover article stitches together multiple studies to create a nearly atomic-level picture of human NPCs.

Paper address: https://www.science.org/doi/pdf/10.1126/science.add2210
The findings build on decades of research including biochemical reconstructions, X-ray crystallography, mass spectrometry, mutagenesis and cell biology. Human NPCs were reconstructed using greatly improved cryo-electron tomography and components were accurately modeled using artificial intelligence techniques. There are other studies that have improved the resolution of single-particle cryo-EM, enabling the visualization of secondary structural elements and residue-level details of vertebrate NPCs. The molecular assemblage enriches our understanding of the architecture of vertebrate and human NPCs—from the old nuclear scaffolding to the connexins that hold the parts together, and from the nuclear membrane anchoring to the cytoplasmic filaments above the central transport channel.
The research results reported here represent a win-win cooperation between experimental structural biology and artificial intelligence, and are another victory for mankind to explore the biological microscopic world. In addition, it also demonstrates that the ongoing revolution in resolution is irreplaceable in our quest to understand the principles of construction and design of macromolecular assemblies.
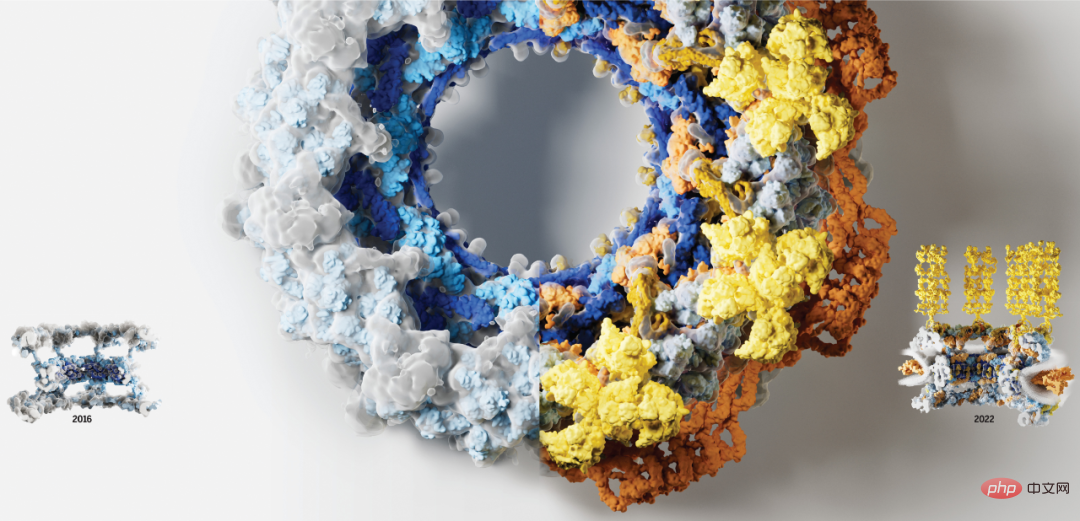
Below is a cross-sectional view of the human nuclear pore complex in 2022, with newly resolved components including the symmetric core (orange) and cytoplasmic filaments (yellow):
Five research papers
Paper 1: "Architecture of the cytoplasmic face of the nuclear pore"

Paper address: https://www.science.org/doi/10.1126/science.abm9129
The nuclear pore complex (NPC) is the only bidirectional channel for nucleocytoplasmic transport. Despite recent progress in elucidating the symmetric core structure of the NPC, the asymmetrically distributed cytoplasmic surface that is a hotspot for mRNA export and nucleoporin-related diseases remains elusive.
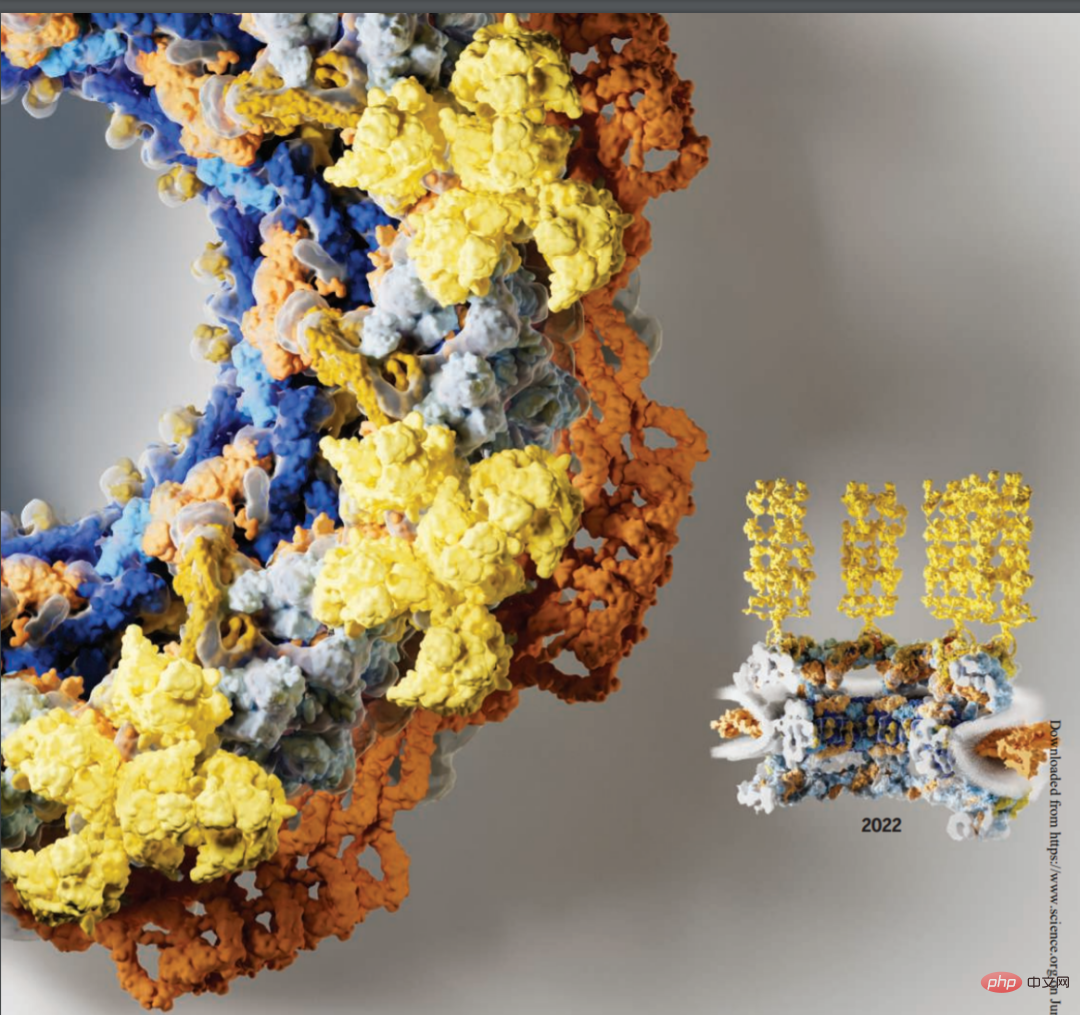
Researchers from Caltech and other institutions report a composite structure of the human cytoplasmic plane obtained by combining biochemical reconstruction, crystal structure determination, cryo-electron tomography reconstruction and physiological verification. While species-specific motifs anchor an evolutionarily conserved, ~540 kilodalton heterohexameric cytoplasmic filament nucleoporin complex above the central transport channel, attachment of the NUP358 pentameric bundle depends Bicyclic arrangement in the coat nucleoporin complex. The complex structures they reveal and their predictive power provide a rich basis for elucidating the molecular basis of mRNA export and nucleoporin diseases.
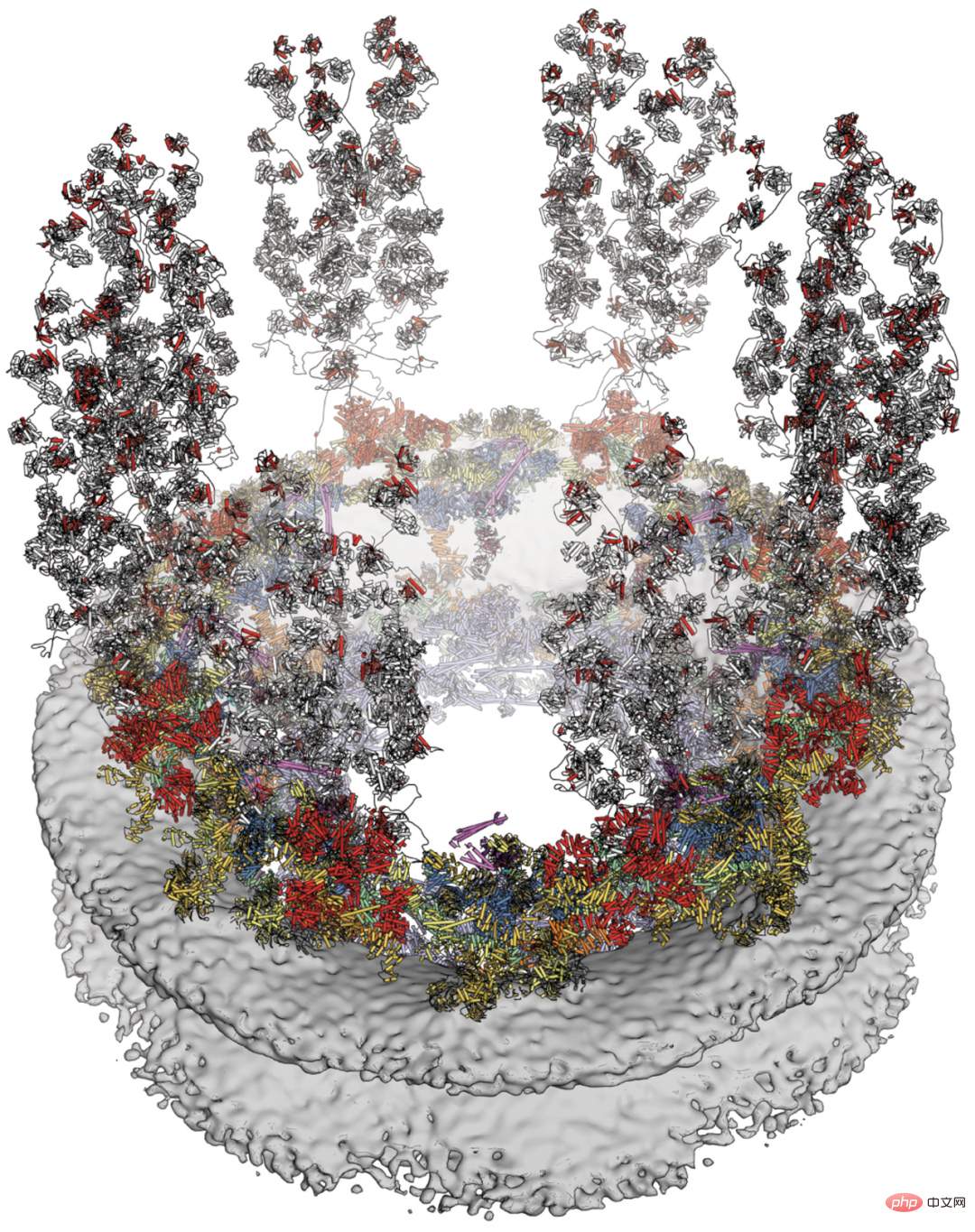
Cytoplasmic face of human NPC.
##Paper 2: "Architecture of the linker-scaffold in the nuclear pore"

Paper address: https://www.science.org/doi/10.1126/science.abm9798
Although The arrangement of structured scaffolding nucleoporins in the symmetric core of NPCs has been determined, but their cohesion through multivalent unstructured linker nucleoporins remains elusive.
By combining biochemical reconstruction, high-resolution structure determination, cryo-electron tomography reconstruction, and physiological validation, Caltech researchers elucidated the evolutionarily conserved joint-scaffold structure that resulted in The human NPC has a near-atomic composite structure core with approximately 64 megadalton symmetry. While joints typically serve a rigid role, the NPC's joint scaffold provides the necessary plasticity and robustness for the reversible contraction and expansion of its central transport channels and the emergence of lateral channels. Their results significantly advance the structural characterization of the NPC symmetry core and lay the foundation for future functional studies.
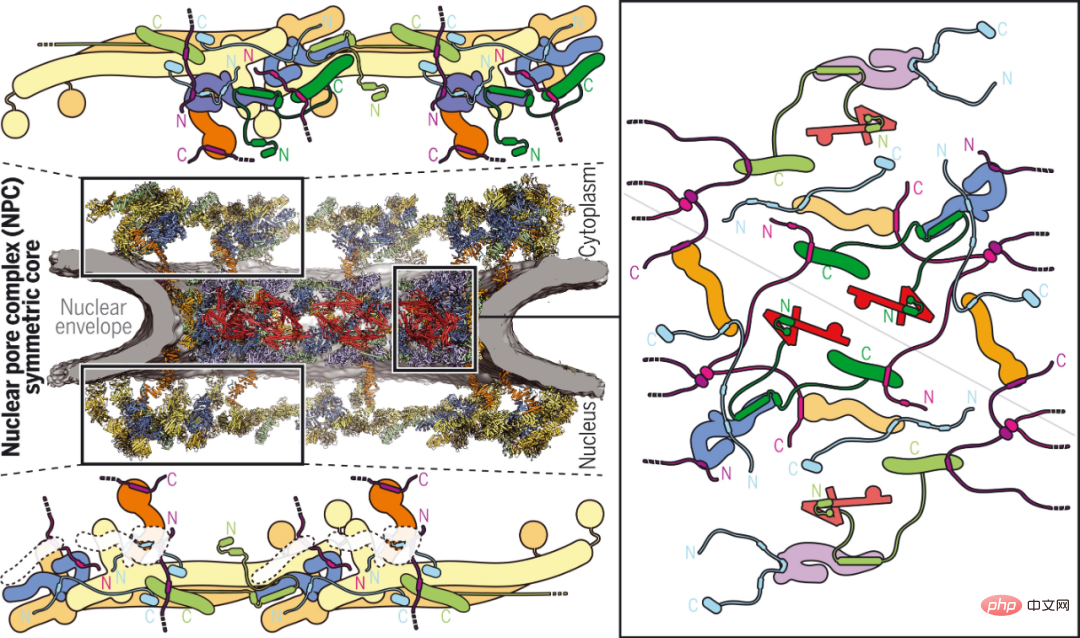
Joint scaffolding structure of the human NPC symmetry core.
Paper 3: "AI-based structure prediction empowers integrative structural analysis of human nuclear pores"

Paper address: https://www.science.org/doi/10.1126/science.abm9506
Although nuclear pore complexes (NPCs) mediate nucleocytoplasmic transport, their intricate 120-megadalton architecture remains incompletely understood. Researchers at the Max Planck Institute for Biophysics and others report a 70-megadalton model of a human NPC scaffold with explicit membranes and multiple conformational states.
They combine AI-based structural predictions with in situ and cellular cryo-electron tomography, comprehensive modeling. The results show that linker nucleoporins organize scaffolds within and between subcomplexes to build higher-order structures. Microsecond-long molecular dynamics simulations show that the scaffold is not required to stabilize the fusion of the inner and outer nuclear membranes, but rather to enlarge the central pore. They illustrate how AI-based modeling can be combined with in situ structural biology to understand subcellular structures across levels of spatial organization.
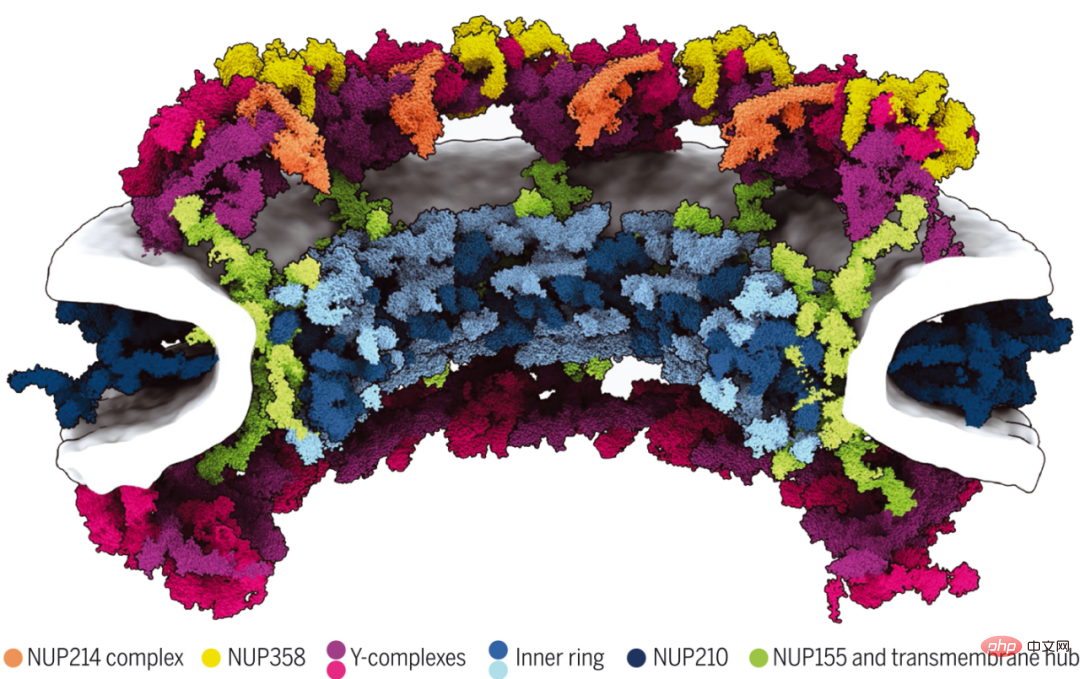
70 MegaDalton model of the human NPC scaffold architecture.
Paper 4: "Structure of the cytoplasmic ring of the Xenopus laevis nuclear pore complex"

Paper address: https://www.science.org/doi/10.1126/science.abl8280
Westlake University and Tsinghua University Single-particle cryo-electron microscopy reconstruction of the cytoplasmic ring subunit of Xenopus laevis NPC at 3.7-4.7 angstrom resolution. Of these, the structure of the amino-terminal domain of Nup358 was solved to 3.0 Å, which facilitated the identification of five Nup358 molecules in each cytoplasmic ring subunit.
The researchers' final model of the cytoplasmic ring subunit includes five Nup358, two Nup205, and two Nup93 molecules, as well as two previously characterized Y complexes. The carboxyl-terminal fragment of Nup160 serves as an organizing center for the apex of each Y complex. Structural analysis reveals how Nup93, Nup205, and Nup358 promote and enhance the assembly of a cytoplasmic ring scaffold formed primarily by two layers of Y complexes.
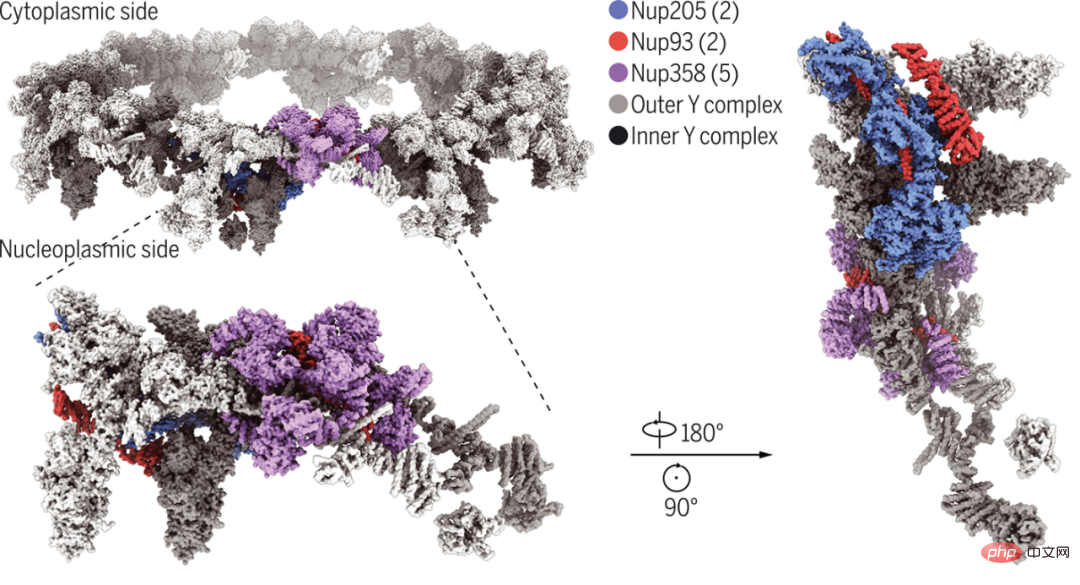
## Cryo-EM structure of the bilayered cytoplasmic ring of Xenopus laevis NPC.
Paper 5: "Structure of cytoplasmic ring of nuclear pore complex by integrative cryo-EM and AlphaFold"

Paper address: https://www.science.org/doi/10.1126/science.abm9326
Researchers from Harvard Medical School and other institutions used single-particle cryo-electron microscopy and AlphaFold prediction to determine a nearly complete NPC cytoplasmic ring structure from Xenopus laevis oocytes. Specifically, they used AlphaFold to predict the structure of nucleoporins and fit the medium-resolution map using prominent secondary structure density as a guide.
Additionally, certain molecular interactions were further established or confirmed through complex predictions using AlphaFold. The researchers identified five binding modes for Nup358, the largest NPC subunit with Phe-Gly repeats for transport. They predicted that Nup358 contains a coiled-coil domain that provides activity to help it serve as a nucleation center for NPC formation under certain conditions.
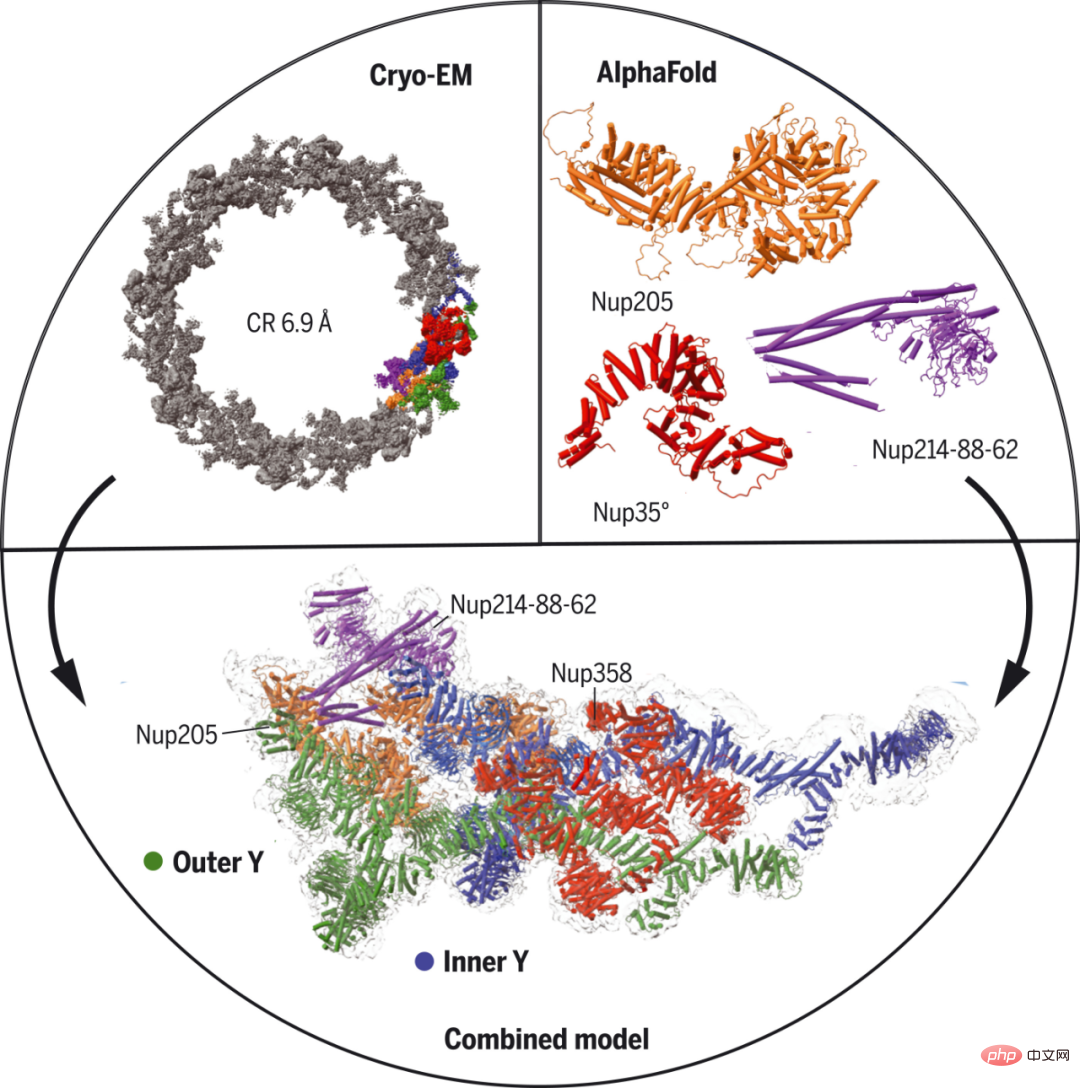
## Cryo-EM structure of Xenopus NPC cytoplasmic loops.
The above is the detailed content of Shi Yigong and other teams appear on the cover of Science: AI and cryo-electron microscopy reveal the 'atomic level' NPC structure, a breakthrough in life sciences. For more information, please follow other related articles on the PHP Chinese website!

Hot AI Tools
Undresser.AI Undress
AI-powered app for creating realistic nude photos
AI Clothes Remover
Online AI tool for removing clothes from photos.

Undress AI Tool
Undress images for free

Clothoff.io
AI clothes remover
Video Face Swap
Swap faces in any video effortlessly with our completely free AI face swap tool!

Hot Article

Hot Tools

Notepad++7.3.1
Easy-to-use and free code editor

SublimeText3 Chinese version
Chinese version, very easy to use

Zend Studio 13.0.1
Powerful PHP integrated development environment

Dreamweaver CS6
Visual web development tools

SublimeText3 Mac version
God-level code editing software (SublimeText3)

Hot Topics
 WorldCoin (WLD) price forecast 2025-2031: Will WLD reach USD 4 by 2031?
Apr 21, 2025 pm 02:42 PM
WorldCoin (WLD) price forecast 2025-2031: Will WLD reach USD 4 by 2031?
Apr 21, 2025 pm 02:42 PM
WorldCoin (WLD) stands out in the cryptocurrency market with its unique biometric verification and privacy protection mechanisms, attracting the attention of many investors. WLD has performed outstandingly among altcoins with its innovative technologies, especially in combination with OpenAI artificial intelligence technology. But how will the digital assets behave in the next few years? Let's predict the future price of WLD together. The 2025 WLD price forecast is expected to achieve significant growth in WLD in 2025. Market analysis shows that the average WLD price may reach $1.31, with a maximum of $1.36. However, in a bear market, the price may fall to around $0.55. This growth expectation is mainly due to WorldCoin2.
 What does cross-chain transaction mean? What are the cross-chain transactions?
Apr 21, 2025 pm 11:39 PM
What does cross-chain transaction mean? What are the cross-chain transactions?
Apr 21, 2025 pm 11:39 PM
Exchanges that support cross-chain transactions: 1. Binance, 2. Uniswap, 3. SushiSwap, 4. Curve Finance, 5. Thorchain, 6. 1inch Exchange, 7. DLN Trade, these platforms support multi-chain asset transactions through various technologies.
 Why is the rise or fall of virtual currency prices? Why is the rise or fall of virtual currency prices?
Apr 21, 2025 am 08:57 AM
Why is the rise or fall of virtual currency prices? Why is the rise or fall of virtual currency prices?
Apr 21, 2025 am 08:57 AM
Factors of rising virtual currency prices include: 1. Increased market demand, 2. Decreased supply, 3. Stimulated positive news, 4. Optimistic market sentiment, 5. Macroeconomic environment; Decline factors include: 1. Decreased market demand, 2. Increased supply, 3. Strike of negative news, 4. Pessimistic market sentiment, 5. Macroeconomic environment.
 Aavenomics is a recommendation to modify the AAVE protocol token and introduce token repurchase, which has reached the quorum number of people.
Apr 21, 2025 pm 06:24 PM
Aavenomics is a recommendation to modify the AAVE protocol token and introduce token repurchase, which has reached the quorum number of people.
Apr 21, 2025 pm 06:24 PM
Aavenomics is a proposal to modify the AAVE protocol token and introduce token repos, which has implemented a quorum for AAVEDAO. Marc Zeller, founder of the AAVE Project Chain (ACI), announced this on X, noting that it marks a new era for the agreement. Marc Zeller, founder of the AAVE Chain Initiative (ACI), announced on X that the Aavenomics proposal includes modifying the AAVE protocol token and introducing token repos, has achieved a quorum for AAVEDAO. According to Zeller, this marks a new era for the agreement. AaveDao members voted overwhelmingly to support the proposal, which was 100 per week on Wednesday
 How to win KERNEL airdrop rewards on Binance Full process strategy
Apr 21, 2025 pm 01:03 PM
How to win KERNEL airdrop rewards on Binance Full process strategy
Apr 21, 2025 pm 01:03 PM
In the bustling world of cryptocurrencies, new opportunities always emerge. At present, KernelDAO (KERNEL) airdrop activity is attracting much attention and attracting the attention of many investors. So, what is the origin of this project? What benefits can BNB Holder get from it? Don't worry, the following will reveal it one by one for you.
 What are the hybrid blockchain trading platforms?
Apr 21, 2025 pm 11:36 PM
What are the hybrid blockchain trading platforms?
Apr 21, 2025 pm 11:36 PM
Suggestions for choosing a cryptocurrency exchange: 1. For liquidity requirements, priority is Binance, Gate.io or OKX, because of its order depth and strong volatility resistance. 2. Compliance and security, Coinbase, Kraken and Gemini have strict regulatory endorsement. 3. Innovative functions, KuCoin's soft staking and Bybit's derivative design are suitable for advanced users.
 The top ten free platform recommendations for real-time data on currency circle markets are released
Apr 22, 2025 am 08:12 AM
The top ten free platform recommendations for real-time data on currency circle markets are released
Apr 22, 2025 am 08:12 AM
Cryptocurrency data platforms suitable for beginners include CoinMarketCap and non-small trumpet. 1. CoinMarketCap provides global real-time price, market value, and trading volume rankings for novice and basic analysis needs. 2. The non-small quotation provides a Chinese-friendly interface, suitable for Chinese users to quickly screen low-risk potential projects.
 Rexas Finance (RXS) can surpass Solana (Sol), Cardano (ADA), XRP and Dogecoin (Doge) in 2025
Apr 21, 2025 pm 02:30 PM
Rexas Finance (RXS) can surpass Solana (Sol), Cardano (ADA), XRP and Dogecoin (Doge) in 2025
Apr 21, 2025 pm 02:30 PM
In the volatile cryptocurrency market, investors are looking for alternatives that go beyond popular currencies. Although well-known cryptocurrencies such as Solana (SOL), Cardano (ADA), XRP and Dogecoin (DOGE) also face challenges such as market sentiment, regulatory uncertainty and scalability. However, a new emerging project, RexasFinance (RXS), is emerging. It does not rely on celebrity effects or hype, but focuses on combining real-world assets (RWA) with blockchain technology to provide investors with an innovative way to invest. This strategy makes it hoped to be one of the most successful projects of 2025. RexasFi