Technology peripherals
Technology peripherals
 AI
AI
 How will data science and artificial intelligence change the healthcare industry?
How will data science and artificial intelligence change the healthcare industry?
How will data science and artificial intelligence change the healthcare industry?
Data science, machine learning, and artificial intelligence have the potential to transform the healthcare industry in profound ways. In this interview, Dr. Shez Partovi, Chief Medical, Innovation and Strategy Officer of Royal Philips, introduced us to the significant role these technologies play in improving patient treatment outcomes and diagnosing diseases.
The conversation included the following topics:
- About health technology company Philips.
- The role of data in healthcare transformation.
- The need to rethink data analytics in healthcare transformation.
- Data collection and data sources in healthcare transformation.
- Connecting data science applications in healthcare to improved patient outcomes.
- Create incentives for data sharing in healthcare.
- Choose the right problem to solve in data science.
- Avoiding bias in data-centric healthcare.
- Patient lockdown based on healthcare data silos.
- Who is responsible for poor data, algorithms and patient outcomes.
- The future of data and artificial intelligence in healthcare;
In March 2021, Shez officially served as the chief innovation and strategy officer of Royal Philips, a leading global medical technology company, responsible for leading Chief Technology Office, Research, HealthSuite Platform, Chief Medical Office, Product Engineering, Experience Design and Strategy, etc. At the same time, the Innovation and Strategy organization also works with operating businesses and markets to guide the company's strategy to meet customer needs and advance the business's growth and profitability goals.
It is reported that Partovi’s career began in 1998 and served as a neuroradiologist at the Barrow Neurological Institute and continued in clinical practice until 2013. Since then, Partovi has worked for DignityHealth, the fifth largest medical system in the United States, for 20 years. In 2018, he joined Amazon and served as the global head of business development for healthcare, life sciences and medical devices, responsible for Amazon's global marketing efforts.
In addition to his medical training at McGill University in Montreal, he has a postgraduate degree in computer science. He helped found the Department of Biomedical Informatics at Arizona State University, where he served as a clinical professor for three years.
Excerpts from the interview:
About the health technology company Philips
Michael Krigsman (moderator): Data science and artificial intelligence are transforming the healthcare industry. Now let’s ask ShezPartovi, chief innovation and strategy officer of Royal Philips, to give us a brief introduction.
ShezPartovi: Seven years ago, Philips decided to divest all businesses except health care, and now it is completely a health technology company. That said, while you may still see Philips lighting, it's really just listed for production. Philips itself is now a 100% health technology company focused on the continuum of care, including home, outpatient, inpatient and more.
So, now when it comes to Philips, think of it as a health technology company. It's a 130-year-old "startup" company because it only really transformed itself 10 years ago, so it feels like a 130-year-old startup.
MichaelKrigsman: As far as I know, you are a doctor. Now you are chief innovation and strategy officer at Philips. Can you briefly describe your role, what you do and what you focus on?
ShezPartovi: I have one of the best jobs in the world because I started Strategic matters. I get to work with customers to understand their unmet needs and, starting from the customer’s problems, work with colleagues to develop strategies that allow Philips to truly solve those problems for customers. This is what the strategic aspect means.
And then in terms of innovation, when we listen to our customers and their problems, we have to be clear about how to innovate from their perspective to solve these problems? The entire innovation community within Philips is part of my responsibility, and we What that does is listen to customer needs, look at market trends and then explore technologies that we have or outside of Philips and integrate them to be able to meet customer needs.
The core of this work is to listen to customers, get signals, formulate strategies, and then communicate them to the innovation team: "How do we creatively meet customer needs? How do we innovate from their perspective?" And then take these Put your ideas into practice. This is the team I lead, and I think it’s the best job I’ve ever had.
The role of data in healthcare transformation
Michael Krigsman: I know a lot of your work is focused on data. Can you explain its role in healthcare transformation?
ShezPartovi: We are doing a lot of data creation and data generation in healthcare right now. Of course, the cynics will say, "Well, yeah, most of that is just for the billing side."
Despite this view, it is also true that the vast majority of data is now digital. For example, when you use a clipboard to fill out content online, it means the data has been digitized. The mechanisms and processes of digitalization are not necessarily seamless and frictionless, and there may be many repetitive and trivial tasks.
When people talk about clinician burnout, physician and nursing burnout, part of the reason is that while we're doing digital transformations, they're not necessarily doing it in a frictionless way, they often don't Workflow awareness and is repetitive. This is not the best digitalization process.
Going back to the issue of data creation and generation, some would argue that we are not using this data meaningfully in a very surprising way. We may have a wealth of data but a lack of insight.
A) We create data in a friction-filled way. B) Unfortunately, we don't really create powerful insights from this data. From my perspective, that's what we need to work on.
Rethinking the Need for Data Analytics in Healthcare Transformation
Michael Krigsman: What led you to ask the two basic questions above?
ShezPartovi: We face some challenges. First, the data generated is still more application-centric, which means it is in silos. I remember when I worked in the health system, we had about 1,500 applications. Now imagine you're digitizing data, and that data exists in an application environment, but you have 1,500 applications.
Second, there is a lack of data mobility, which means that although you have the data digitized and stored on disk, you are not necessarily able to combine it into an environment and derive insights from it. With so many siled environments, we have a lot of work to do to bring all the data together into a common context from which insights can be generated.
Of course, technology is also advancing. Philips has an environment called "Health Suite" which is related to data mobility. It brings in data from hundreds of different sources, combines it, and then derives insights from it.
This is what we do. In fact, many health systems currently struggle with integrating data into a common environment.
Data collection and data sourcing in healthcare transformation
Michael Krigsman: It appears that data mobility and interoperability remain bottlenecks in many areas of digital activity . Is that so?
Shez Partovi:Yes. In fact, you should think of data interoperability as having two dimensions: syntactic interoperability where you just share data, and semantic interoperability where you share meaning.
It is true that we still have many data silos, but it is undeniable that we have also made great progress in data interoperability. As more interoperability occurs, some organizations have created fluidity, and with it the vision of moving from data to information, from information to knowledge, and from knowledge to insights. This is where artificial intelligence can come into play.
Michael Krigsman: How do you move from the data-rich you just described to data-driven?
ShezPartovi:I can give you an example An example of moving from data to information, information to knowledge, knowledge to insight. Let's start with a single data point, such as blood glucose. If your single blood sugar level is about 140 milligrams per deciliter, that's high. But on the other hand, is it because that person just ate? Is it fasting blood sugar? Is it non-fasting blood sugar? So, it's just a data point. Useful, but not yet insightful.
But if what I tell you is an upward trend in blood sugar, then this is information. Information is a trend, and this trend is rising, which means there may be some problems with your body.
If we look further into the patient's history and understand that they may have early stage diabetes, this is knowledge. But today, to achieve the Triple Aim—improving quality, lowering costs, and improving experience—in a positive way, we need to understand what health systems need, what clinicians, doctors, nurses, and organizations need, and they want more than just data , information or knowledge.
They want to answer the following questions: How likely is it that this patient you showed me has prediabetes? How likely is it that they will develop congestive heart failure within the next 18 months? How big? How likely are they to develop a diabetic foot ulcer in the next two years? This kind of prediction, this insight into the future, is the real opportunity. When you bring data together and are able to use it to build machine learning models and use AI, you are truly using data to drive insights for your organization.
This is the main line:From data to information to knowledge, to observation, and finally to something feasible for me. The best service I can provide to this patient at this point in time of service. Michael Krigsman: How do you connect all of this to patient outcomes? Or, if we What are the advantages of having this kind of interoperability? Clinician or patient clinical experience or consumer experience. For example, start with what I think is probably the easiest cost reduction. One of the things that using artificial intelligence in conjunction with data can do is help with what are called real-world operational predictions, like, how many staff do I need to staff my emergency department next Friday night? What's the patient flow rate? Can I predict the flow of patients coming into the hospital so I can adjust staffing levels? And by the way, that affects the quality of care because if you're short-staffed, it's going to be a challenge. Right-sizing has a positive impact on both cost and quality of care. For example, use ADT flow (admission, discharge, transfer flow) to build a model to provide predictions for patient flow into the hospital, thereby helping you achieve reasonable staffing, which will not only affect the patient experience, It also affects the clinician experience. Because if there is insufficient manpower, it will undoubtedly affect the quality of care. This is exactly what Philips is currently doing. The above is the operational forecast. Let’s talk about the clinical forecast mentioned before. I use diabetes as an example to predict the likelihood of diabetic foot ulcers or heart disease. In fact, there are many examples of clinical prediction. For example, you can use artificial intelligence and machine learning to read radiology images to identify or predict abnormalities. If the algorithm determines that an image should be processed as quickly as possible because of its impact on quality of care, radiologists should review it immediately and take proactive action. Rather than arranging the images in the order in which they were taken, the algorithm places images with anomalies first. Only detection first and treatment first can produce positive treatment results. At Philips, these are the most common use cases for AI and ML to improve patient outcomes. Creating incentives for data sharing in healthcare Shez Partovi: The data belongs to the health system and the software company doesn't actually own it. For example, Philips does not own this data, we are essentially just data stewards. However, I do know that there are software companies in some countries around the world (I won’t name them because I don’t want to say bad things about them) that actually own this data. Therefore, it can also be said that your argument is correct. But at least in the United States, it’s not correct to say “I refuse to share data.” Information blocking rules will prohibit this. Can you tell us the types of data that need to be aggregated? ShezPartovi: If you are thinking about AI and machine learning, clinical For forecasting and operational forecasting, you need to start with the problem to understand what data is needed. Take Google Maps, for example, if you remember, there was a time when it just showed you directions and elapsed times in red heatmaps. Later, it started showing time spent biking, time spent walking, best routes, and more. Depending on the predictions and value they want to provide, they are collecting more and more data. Now back to the enterprise itself, when considering what data needs to be collected to create a model, we also need to start from the problem. Say you want to predict length of stay to maintain an appropriate and efficient scale of care. Then all you probably need is an ADT stream to predict dwell time. On the other hand, if you're trying to predict whether a person has a specific disease or a specific cancer, you might need imaging, blood values, EHR (electronic health record data) data. In summary, you need to start with the problem statement, understand what you are trying to predict, and the tools you want to provide clinicians or operations teams, and then work backwards to see what data you need to build this for You provide the predictive model. Choosing the right problem to solve in data science Shez Partovi: Every organization either has operations people trying to solve operational problems, or it may have a lean team. Lean teams were really popular a while ago. Now there is a transformation team. Although they have different names, they are both looking around for problems that need to be solved. And of course there's the clinical excellence team and the operational excellence team. However, they may be called different things in your organization. If you attend their steering committee meetings, you will find that they probably know the problems they need to solve and the headaches they have. In fact, this is what customers tell them. So, I would recommend starting with these teams that are already running programs, for example, the chief nursing officer or chief medical officer is running a clinical excellence program; the chief operating officer is running an operational lean program or excellence program. They are trying to solve some challenges. So they have the data to build machine learning models as tools to solve these problems. I would say that if I were the chief marketing officer (CMO) of a health system, I would certainly have many problems to solve, but I would start with those that are already being studied. Also, consider using AI and ML as tools for these teams. Michael Krigsman: In other words, address the immediate practical problems you may face, whether clinical Or surgery. Right? ShezPartovi: Of course. I mean I'm very practical now. It might be aligned with organizational KPIs, aligned with team KPIs. That's really the easiest, most direct place to start, with these things. Michael Krigsman:Are the challenges of becoming a more data-centric healthcare system that utilizes data more effectively, a technology bias toward the operational side? ShezPartovi:First of all, of course you need to digitize the data. When it comes to data, there are three Vs: volume, variety, and veracity. To truly create a model that serves as a conservation tool, you need to implement these qualities. Because large-scale helps eliminate bias; diversity creates better machine learning models; authenticity restores the truth of the data. This is the first step. Next, you need to actually train a model, you need to label the data, and validate the model. Additionally, you will need to decide whether to apply for FDA review (as Philip did), and not only will you need to verify it, but you will also need to meet certain requirements. Conduct results studies to prove this is the case. Again, this is more on the supplier side. Internally, for operations, you don't need to do that. Data (massification, diversity, authenticity), labeling, machine learning, modeling, testing and validation, etc., all these activities require organizations to collaborate with health technology companies. For some complex academic medical centers, they will also contact the university to find the talent they need to help. When you ask me what the barriers are, it depends on whether you're implementing tools that you might get from Philips, or if you want to build those yourself. In that case, you might partner with a health tech company that can help you, or some kind of company that can help you, or you might decide to build an in-house capability to do that. The tools are there. But bringing it all together requires competency, training and upskilling. So you either build it in-house or work with your partners. Michael Krigsman: How can an organization create an enterprise-wide view when data comes from various systems of record? After all, it comes from different software vendors and is essentially a different system. ShezPartovi: You want your data to be in an environment where it all comes together. Technically, at least, you have to consider that you do need a holding area, call it a "data lake", or whatever you want to call it, a healthy data space. The questioner mentioned the issue of visualization, which I think is important. Above I talked about data, information, knowledge, and insight, and if you remember, visualization is the term I use for turning data into information. People tend to associate visualization with “showing dashboards and charts.” But I think the more powerful thing, and probably what's hinted at in the question, is how can I create insights from this data, which can get a much higher ROI than simple visualization. By the way, I think you do need some kind of data lake environment, preferably in the cloud, because if you're going to run machine learning models, you don't want to buy an expensive GPU sitting in a data center and just use it for half an hour a day and let it 23.5 hours idle. You can use the cloud and pay based on what you use. In the cloud, you can use the most complex machine learning model training sets, training techniques, and only pay for what you use. If you try to build it in your own data center, you're going to pay a lot for something that you only use a fraction of the time. Never do that. MichaelKrigsman: Tell you a little story about myself, I won’t name a specific healthcare system, but I absolutely will Stick with them. There are many reasons, one of which is that they are great, great doctors, etc. But it also has an information lock, and if I leave their system, the doctor will send me a notification. Wouldn't this inherent information lock-in be detrimental to the data sharing you describe? ShezPartovi: This will definitely make data sharing more difficult. However, many organizations are trying to move away from this status quo. For example, our partner at the University of California, San Francisco (UCSF) is using the common shared environment that I mentioned above, and they're actually taking data from practices outside of the UCSF environment and trying to create a holistic view that enables patients Moving between practices becomes simple and effortless, as does information sharing. Michael Krigsman: If there is bad data, bad algorithms, and bad predictions made as a result, Who is responsible? Shez Partovi:At Philips we continue to believe in this being a tool to help clinicians make decisions, but ultimately you want to let the clinician be the final decision maker . Back to the question itself, first of all, philosophically speaking, at least from our perspective, we are looking at how to create a tool that is transparent, unbiased and improves the experience, which can also help clinicians complete They work just like a blood test or any other test. By the way, any test can have false positives or false negatives. Clinicians make decisions based on their verification and comprehensive considerations, unlike algorithms that diagnose themselves. Whether it’s data, algorithms or predictions, they are simply tools to help clinicians make decisions. Then, when it comes to data bias, I will add 1V, verification (validation) to the previous 3V (Variety, Diversity, and Authenticity). Of course, the process of creating an algorithm involves this massification, diversity, authenticity, and then validation. The fact that as clinicians we all view health care as local care means that a disease that is endemic in one area may not be endemic in another. I have trained in both Canada and the United States. I can tell you that a particular chest X-ray in Canada was tuberculosis, and the same finding I had in training in the United States was pneumococcidioidomycosis. They are different. But that's because health care is local. The algorithm needs to be fine-tuned according to the deployment environment. There will not be a universal algorithm for the world, let alone the United States. Healthcare is local. Training needs to be fine-tuned locally. Michael Krigsman: You make a very provocative point that models need to be localized or reflect local conditions. Who should be responsible for creating these models? ShezPartovi: Algorithms can be fine-tuned - and we do too. Therefore, a model can be trained and fine-tuned "generally" or even deployed in the background in an environment before going into production, and then continue training after deployment. By definition, it becomes localized through its implementation and continued use. Michael Krigsman:Will these models typically be provided by software vendors, healthcare systems, or companies like Philips? Who will provide these models? Shez Partovi: All of the above are available. Of course, Philips also develops models, and we actually have an environment called an "AIManager" where you can put our models and use it. Organizations can also build their own models and put them into AI Manger for use. Currently, there are many young companies doing this. I think any company that has access to data can use good data to build models. Michael Krigsman: You mean that the local model is a way to reduce the internal bias of the model, am I understanding it correctly? Shez Partovi:Yes, it does help reduce bias. After the trained and verified model enters the local and starts to be used, it is considered a local optimization and adjustment model. Michael Krigsman: Where will data and AI in healthcare go in the next few years? ? ShezPartovi: When you take some body tissue or blood for testing, a data stream will be generated. You can obtain the data and run the algorithm on it as a test. Well, just like you draw blood and test the blood, you can have your data flowing through the veins and arteries of the health system. You can take that data and apply algorithms to it. Clinicians use ordering algorithms as a test. Yes, there are background algorithms that are always running. But some algorithms may require a lot of computing power. Such algorithms can actually end up costing you money because you're using computing power to run them. I think that over time, clinicians will order algorithms the same way they order tests. Michael Krigsman: How long do you predict it will take for this to happen? Shez Partovi: I think we may see some early signs in five to ten years . Michael Krigsman: How do we ensure that data science is used to improve patient care, not just improve profits? And technology is very expensive, how should that be considered? ShezPartovi: People should correctly view the comprehensive significance of AI and ML technology in healthcare to improve quality, reduce costs and improve experience. In fact, it can be seen that cost accounts for only one-third of these factors. We should focus on all aspects of the Triple Aim, not just cost reduction. I did say before that "data science is used to improve operational efficiency", but in my opinion, in some cases, improving operational efficiency is to improve care services, because for example, understaffing can lead to low quality. These factors are all tied together and I don't want it to seem like they are separate. MichaelKrigsman: What advice do you have for healthcare managers looking at this changing environment? They know they need to adapt, but it's going to be very tough for them as they endure There's so much financial pressure, regulatory pressure, all kinds of different pressures. ShezPartovi: I have a deep understanding of this in the early stages of cooperation with other organizations. I know it sounds selfish because I'm representing Philips here, but if I were the CMO, I would do training and upskilling and a lot of other things. My suggestion to administrators is to find the best solutions from peers for their own problems and introduce a technology partner to see how AI ML can be applied to this problem together with this partner. . This is how I conduct myself as an administrator. Michael Krigsman: What do you want policymakers to understand about this changing world of health care? Shez Partovi: Policymakers should and need to know. AI and ML play an important role in advancing the Triple Aim. So, in my opinion, in this day and age, AIML can improve quality, reduce costs, and improve the experience for patients and clinicians. Policymakers should look at how to advance data science adoption and remove barriers to AI and ML, as the net effect of doing so is what other teams want. These three goals are interrelated, and we should figure out how to advance practice through policy. Linking Data Science in Healthcare to Improved Patient Outcomes
Huge amounts of data are held in a very small number of market-leading applications. Why would a monopoly have an incentive to share this data? In other words, wouldn’t the market power of software and infrastructure affect the kind of data sharing you describe?
How do you ensure you choose the right problem to solve?
Avoiding Bias in Data-Centric Healthcare
Patient Lockdown Based on Healthcare Data Silos
Who is Responsible for Bad Data, Algorithms, and Patient Outcomes
The Future of Data and AI in Healthcare
The above is the detailed content of How will data science and artificial intelligence change the healthcare industry?. For more information, please follow other related articles on the PHP Chinese website!

Hot AI Tools

Undresser.AI Undress
AI-powered app for creating realistic nude photos

AI Clothes Remover
Online AI tool for removing clothes from photos.

Undress AI Tool
Undress images for free

Clothoff.io
AI clothes remover

AI Hentai Generator
Generate AI Hentai for free.

Hot Article

Hot Tools

Notepad++7.3.1
Easy-to-use and free code editor

SublimeText3 Chinese version
Chinese version, very easy to use

Zend Studio 13.0.1
Powerful PHP integrated development environment

Dreamweaver CS6
Visual web development tools

SublimeText3 Mac version
God-level code editing software (SublimeText3)

Hot Topics
 1377
1377
 52
52
 Bytedance Cutting launches SVIP super membership: 499 yuan for continuous annual subscription, providing a variety of AI functions
Jun 28, 2024 am 03:51 AM
Bytedance Cutting launches SVIP super membership: 499 yuan for continuous annual subscription, providing a variety of AI functions
Jun 28, 2024 am 03:51 AM
This site reported on June 27 that Jianying is a video editing software developed by FaceMeng Technology, a subsidiary of ByteDance. It relies on the Douyin platform and basically produces short video content for users of the platform. It is compatible with iOS, Android, and Windows. , MacOS and other operating systems. Jianying officially announced the upgrade of its membership system and launched a new SVIP, which includes a variety of AI black technologies, such as intelligent translation, intelligent highlighting, intelligent packaging, digital human synthesis, etc. In terms of price, the monthly fee for clipping SVIP is 79 yuan, the annual fee is 599 yuan (note on this site: equivalent to 49.9 yuan per month), the continuous monthly subscription is 59 yuan per month, and the continuous annual subscription is 499 yuan per year (equivalent to 41.6 yuan per month) . In addition, the cut official also stated that in order to improve the user experience, those who have subscribed to the original VIP
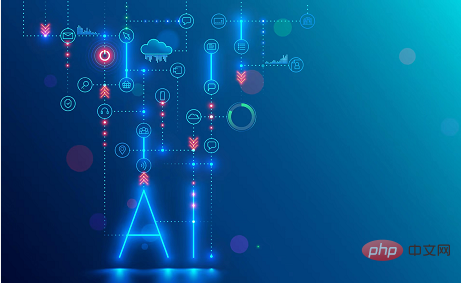
 Context-augmented AI coding assistant using Rag and Sem-Rag
Jun 10, 2024 am 11:08 AM
Context-augmented AI coding assistant using Rag and Sem-Rag
Jun 10, 2024 am 11:08 AM
Improve developer productivity, efficiency, and accuracy by incorporating retrieval-enhanced generation and semantic memory into AI coding assistants. Translated from EnhancingAICodingAssistantswithContextUsingRAGandSEM-RAG, author JanakiramMSV. While basic AI programming assistants are naturally helpful, they often fail to provide the most relevant and correct code suggestions because they rely on a general understanding of the software language and the most common patterns of writing software. The code generated by these coding assistants is suitable for solving the problems they are responsible for solving, but often does not conform to the coding standards, conventions and styles of the individual teams. This often results in suggestions that need to be modified or refined in order for the code to be accepted into the application
 Can fine-tuning really allow LLM to learn new things: introducing new knowledge may make the model produce more hallucinations
Jun 11, 2024 pm 03:57 PM
Can fine-tuning really allow LLM to learn new things: introducing new knowledge may make the model produce more hallucinations
Jun 11, 2024 pm 03:57 PM
Large Language Models (LLMs) are trained on huge text databases, where they acquire large amounts of real-world knowledge. This knowledge is embedded into their parameters and can then be used when needed. The knowledge of these models is "reified" at the end of training. At the end of pre-training, the model actually stops learning. Align or fine-tune the model to learn how to leverage this knowledge and respond more naturally to user questions. But sometimes model knowledge is not enough, and although the model can access external content through RAG, it is considered beneficial to adapt the model to new domains through fine-tuning. This fine-tuning is performed using input from human annotators or other LLM creations, where the model encounters additional real-world knowledge and integrates it
 Seven Cool GenAI & LLM Technical Interview Questions
Jun 07, 2024 am 10:06 AM
Seven Cool GenAI & LLM Technical Interview Questions
Jun 07, 2024 am 10:06 AM
To learn more about AIGC, please visit: 51CTOAI.x Community https://www.51cto.com/aigc/Translator|Jingyan Reviewer|Chonglou is different from the traditional question bank that can be seen everywhere on the Internet. These questions It requires thinking outside the box. Large Language Models (LLMs) are increasingly important in the fields of data science, generative artificial intelligence (GenAI), and artificial intelligence. These complex algorithms enhance human skills and drive efficiency and innovation in many industries, becoming the key for companies to remain competitive. LLM has a wide range of applications. It can be used in fields such as natural language processing, text generation, speech recognition and recommendation systems. By learning from large amounts of data, LLM is able to generate text
 To provide a new scientific and complex question answering benchmark and evaluation system for large models, UNSW, Argonne, University of Chicago and other institutions jointly launched the SciQAG framework
Jul 25, 2024 am 06:42 AM
To provide a new scientific and complex question answering benchmark and evaluation system for large models, UNSW, Argonne, University of Chicago and other institutions jointly launched the SciQAG framework
Jul 25, 2024 am 06:42 AM
Editor |ScienceAI Question Answering (QA) data set plays a vital role in promoting natural language processing (NLP) research. High-quality QA data sets can not only be used to fine-tune models, but also effectively evaluate the capabilities of large language models (LLM), especially the ability to understand and reason about scientific knowledge. Although there are currently many scientific QA data sets covering medicine, chemistry, biology and other fields, these data sets still have some shortcomings. First, the data form is relatively simple, most of which are multiple-choice questions. They are easy to evaluate, but limit the model's answer selection range and cannot fully test the model's ability to answer scientific questions. In contrast, open-ended Q&A
 Five schools of machine learning you don't know about
Jun 05, 2024 pm 08:51 PM
Five schools of machine learning you don't know about
Jun 05, 2024 pm 08:51 PM
Machine learning is an important branch of artificial intelligence that gives computers the ability to learn from data and improve their capabilities without being explicitly programmed. Machine learning has a wide range of applications in various fields, from image recognition and natural language processing to recommendation systems and fraud detection, and it is changing the way we live. There are many different methods and theories in the field of machine learning, among which the five most influential methods are called the "Five Schools of Machine Learning". The five major schools are the symbolic school, the connectionist school, the evolutionary school, the Bayesian school and the analogy school. 1. Symbolism, also known as symbolism, emphasizes the use of symbols for logical reasoning and expression of knowledge. This school of thought believes that learning is a process of reverse deduction, through existing
 SOTA performance, Xiamen multi-modal protein-ligand affinity prediction AI method, combines molecular surface information for the first time
Jul 17, 2024 pm 06:37 PM
SOTA performance, Xiamen multi-modal protein-ligand affinity prediction AI method, combines molecular surface information for the first time
Jul 17, 2024 pm 06:37 PM
Editor | KX In the field of drug research and development, accurately and effectively predicting the binding affinity of proteins and ligands is crucial for drug screening and optimization. However, current studies do not take into account the important role of molecular surface information in protein-ligand interactions. Based on this, researchers from Xiamen University proposed a novel multi-modal feature extraction (MFE) framework, which for the first time combines information on protein surface, 3D structure and sequence, and uses a cross-attention mechanism to compare different modalities. feature alignment. Experimental results demonstrate that this method achieves state-of-the-art performance in predicting protein-ligand binding affinities. Furthermore, ablation studies demonstrate the effectiveness and necessity of protein surface information and multimodal feature alignment within this framework. Related research begins with "S
 Laying out markets such as AI, GlobalFoundries acquires Tagore Technology's gallium nitride technology and related teams
Jul 15, 2024 pm 12:21 PM
Laying out markets such as AI, GlobalFoundries acquires Tagore Technology's gallium nitride technology and related teams
Jul 15, 2024 pm 12:21 PM
According to news from this website on July 5, GlobalFoundries issued a press release on July 1 this year, announcing the acquisition of Tagore Technology’s power gallium nitride (GaN) technology and intellectual property portfolio, hoping to expand its market share in automobiles and the Internet of Things. and artificial intelligence data center application areas to explore higher efficiency and better performance. As technologies such as generative AI continue to develop in the digital world, gallium nitride (GaN) has become a key solution for sustainable and efficient power management, especially in data centers. This website quoted the official announcement that during this acquisition, Tagore Technology’s engineering team will join GLOBALFOUNDRIES to further develop gallium nitride technology. G