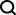Technology peripherals
Technology peripherals
 AI
AI
 With this 'bionic nose', COVID-19 survivors will have the opportunity to 'smell the roses' again
With this 'bionic nose', COVID-19 survivors will have the opportunity to 'smell the roses' again
With this 'bionic nose', COVID-19 survivors will have the opportunity to 'smell the roses' again
One Sunday in 2012, while teaching his 6-year-old son to skateboard, Scott Moorehead fell to the ground. “The back of my skull took the brunt of it,” he said. He spent three days in the intensive care unit, where doctors treated him for multiple skull fractures, massive internal bleeding and damage to the frontal lobe of his brain.
After weeks and months, Moorehead's hearing returned, his headaches disappeared, and so did his irritability and confusion, but he never regained his sense of smell.
The accident permanently severed the nerves from Moorehead's nose to his olfactory bulb at the base of his brain. In addition to his sense of smell, he also lost his basic sense of taste. "Taste comes mostly from smell," he explains. "My tongue can only perceive sweet, salty, spicy and bitter. You could blindfold me and put 10 flavors of ice cream in front of me and I wouldn't know the difference: they all taste a little sweet, except The chocolate is a little bitter."
Moorehead suffered terribly: in addition to the taste of food, he missed the unique smell of his loved ones. On one occasion, he failed to notice a gas leak and did not realize the danger until his wife came home and called the police.
Moorehead's pain isn't unique. Loss of smell can be caused not only by head injuries, but also by exposure to certain toxins and a variety of medical issues, including tumors, Alzheimer's and viral diseases. , like COVID. The sense of smell also typically shrinks with age; in a 2012 study of more than 1,200 adults who had their sense of smell tested, 39 percent of participants aged 80 and older had olfactory dysfunction.
Loss of smell and taste has been a major COVID symptom since the beginning of the pandemic. People with COVID-induced anosmia currently have only three options: wait and see if feeling returns on its own, request steroid medication to reduce inflammation and potentially speed recovery, or start getting retested, something they're exposing themselves to Smell familiar smells every day to promote the recovery of rhinocerebral nerves. Medication is usually effective if patients seek treatment and recover within a few weeks of the onset of symptoms. But even so, these interventions don't work for everyone.
In April 2020, researchers at the VCU Smell and Taste Clinic launched a national survey of adults diagnosed with COVID to determine the prevalence and duration of smell-related symptoms. They followed these people regularly, and last August they published results from people two years after their initial diagnosis. The results were shocking: 38% reported full recovery of their sense of smell and taste, 54% reported partial recovery, and 7.5% reported no recovery at all. "This is a serious quality of life issue," said VCU Clinic Director Evan Reiter.
Regenerating the sense of smell
While many researchers are working on biological methods, such as using stem cells to regenerate odor receptors and nerves, Richard Costanzo believes that hardware implants are the only solution for people who have completely lost their sense of smell. solution. "When those pathways really become unavailable, you have to replace them with technology," he said.
Costanzo, a professor emeritus of physiology and biophysics, founded VCU’s Center for Smell and Taste Disorders in the 1980s, one of the first clinics of its kind in the country. After years of studying smell loss and investigating the possibility of biological regeneration, he began working on hardware solutions in the 1990s.
He has developed a prototype electronic nose: a neuroprosthetic for smell, in which electronic nose sensors transmit their signals to an array of small electrodes taken from a cochlear implant. For people with hearing loss, implants can feed information about sounds into the inner ear and then into the brain. The implant is also sized to fit in the olfactory bulb at the edge of the brain. So, could it also be used to convey information about smells?
Unlike most people with anosmia, Moorehead didn't give up when doctors told him they couldn't regain their sense of smell. As CEO of a mobile phone retail company with stores in 43 states, he has the resources to invest in long-term research. When a colleague told him about the work at VCU, he got in touch and offered a research grant. Since 2015, Moorehead has invested nearly $1 million in the research. He also licensed the technology from VCU and founded a startup called Sensory Restoration Technologies.
When COVID hit, Moorehead saw an opportunity. Although they were far from launching a product to promote, he quickly built a website for the startup. He said: "People are losing their sense of smell. People need to know that we exist!" Neuroprosthetics, such as hearing.
Cochlear implants are the most successful neurotechnology to date, with more than 700,000 devices implanted in ears worldwide. Meanwhile, there are already research institutions developing retinal implants for the blind, but smell and taste have been considered a difficult challenge.
To understand why, we first need to understand the wonderful complexity of the human olfactory system. When the scent of a rose wafts into your nasal passages, odor molecules bind to receptor neurons, sending electrical signals to the olfactory nerve. These nerves travel through bony plates to the olfactory bulb, a small neural structure in the forebrain. From there, the information goes to the amygdala, the part of the brain that controls emotional responses. the hippocampus, a structure involved in memory; and the frontal cortex, which handles cognitive processing.
Odor molecules entering the nose bind to olfactory receptor cells, which send signals through the bones of the lamina cribrosa to the olfactory bulb. From there, the signal is sent to the brain.
These branching neural connections are why smells sometimes hit so hard, bringing to mind happy memories or traumatic events. “The olfactory system has access to parts of the brain that are inaccessible to other senses,” Coelho says. The diversity of brain connections also suggests that stimulating the olfactory system may have other uses, well beyond appreciating food or noticing gas leaks: “It might Can affect mood, memory and cognition."
Biological systems are difficult to replicate for several reasons. The human nose has approximately 400 different types of receptors that detect odor molecules. These receptors work together to allow humans to distinguish a staggering number of odors: a 2014 study estimated the number at 1 trillion. Until now, it has been impractical to put 400 sensors on a chip and attach them to a user's glasses. What's more, researchers don't yet fully understand the olfactory code that stimulates certain combinations of receptors that cause the brain to perceive odors. Fortunately, Costanzo and Coelho knew someone who could solve both problems.
Research Progress in Electronic Noses and Brain Stimulation
Today, electronic noses are used in a variety of industrial, office, and residential settings—if you have a typical carbon monoxide detector in your home, you There is a very simple electronic nose.
"Conventional gas sensors are based on semiconductors such as metal oxides," explains Krishna Persaud, a leading electronic nose researcher and professor of chemoreception at the University of Manchester in the UK. He is the consultant of Costanzo and Coelho. In the most typical electronic nose devices, he said, "when molecules interact with the semiconductor material, the resistance changes and you can measure it." Such sensors have been shrinking over the past two decades, Persaud said. , now they are the size of a microchip. "This makes them very convenient in a small package," he said. In the VCU team's early experiments, they used off-the-shelf sensors from a Japanese company called Figaro.
The problem with such commercially available sensors, Persaud said, is that they can't distinguish between many different odors. That's why he's been researching new materials, such as conductive polymers that are cheap to make, low-power and can be combined into arrays to provide sensitivity to dozens of odors. For neuroprostheses, "in principle, hundreds [of sensors] are feasible," Persaud said.
First-generation products did not allow users to smell hundreds of different scents. Instead, the VCU team originally envisioned including some receptors for safety-related odors, such as smoke and natural gas, as well as some pleasant smells. They can even customize prosthetics to give users scents that are meaningful to them: the smell of bread for a home baker, for example, or the smell of a pine forest for an avid hiker.
Combining this electronic nose technology with the latest neurotechnology is the challenge Costanzo and Coelho are currently facing. While they're working with Persaud to test the new sensor, they're also working with clinicians in Boston on the best ways to send signals to the brain.
The VCU team laid the groundwork through animal experiments. In experiments on rats in 2016 and 2018, the team showed that using electrodes to directly stimulate spots on the surface of the olfactory bulb produced patterns of neural activity deep in the bulb that carried information to other parts of the brain. Researchers call these patterns odor maps. But while the neural activity suggested the mice were sensing something, the mice couldn't tell the researchers what they were smelling.
Otolaryngologist Eric Holbrook often works with patients who require surgery within the sinus cavities. He helped the VCU team conduct preliminary clinical trials.
Their next step is to recruit collaborators who can conduct similar experiments with human volunteers. They started with one of Costanzo's former students, Holbrook, an associate professor of otolaryngology at Harvard Medical School and chief of the Division of Eye, Ear and Nose at Massachusetts. Holbrook spends most of his time operating on people's sinus cavities, including the ethmoid cavity, which lies beneath the cribriform plate, a bony structure that separates olfactory receptors from the olfactory bulbs.
Holbrook discovered in 2018 that placing electrodes on the bone sends electrical impulses to the olfactory bulb. In a trial with awake patients, three of five volunteers reported olfactory perceptions during this stimulation, with reported odors including "oniony," "preservative and sour," and "fruity." But it doesn’t smell good”. While Holbrook sees the trial as a good proof of concept for an olfactory implant system, he says poor conductivity through bone is a significant limiting factor. "If we're going to deliver discrete, independent areas of stimulation," he said, "it can't be through the bone, but needs to be on the olfactory bulb itself."
Placing electrodes on the olfactory bulb will be new territory. "Theoretically," Coelho said, "there are a lot of different ways to get there." Surgeons can go down through the brain, sideways through the eye socket, or up through the nasal cavity to break through the cribriform plate and get to the bulb. Coelho explained that nasal surgeons often perform low-risk surgeries that involve breaching the lamina cribriformis. "What's new is not how to get there or clean up afterward," he said, "but how to keep the foreign object there without causing problems."
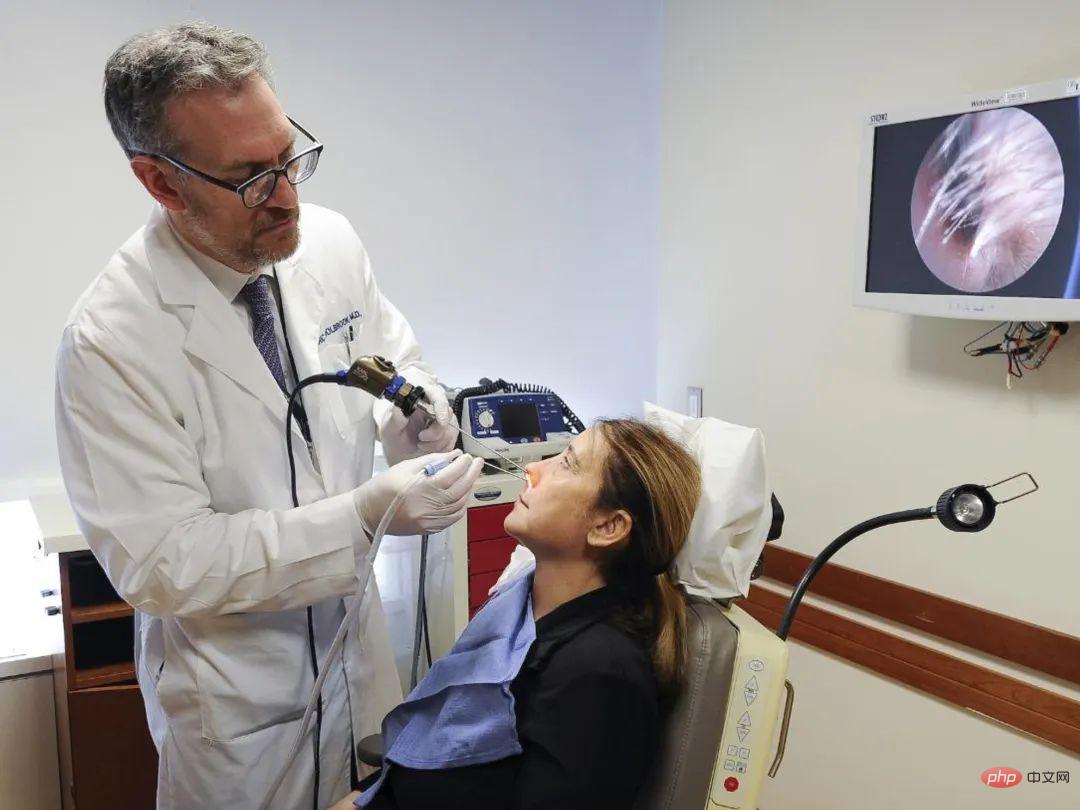
Neurosurgery Dr. Mark Richardson's epilepsy patients volunteered to participate in neuroscience research while they were hospitalized, using implanted electrodes for brain monitoring.
Another strategy is to skip the olfactory bulb entirely and instead stimulate the "downstream" part of the brain that receives signals from the olfactory bulb. Supporting this approach is Holbrook's former student Mark Richardson, chief of functional neurosurgery at Massachusetts General Hospital. Richardson often keeps epilepsy patients in the hospital for several days with electrodes in their brains so doctors can determine which brain areas are involved in their seizures and plan surgical treatments. However, while these patients wait, they are often recruited for neuroscience research.
To facilitate Costanzo and Coelho's research, Richardson's team asked epilepsy patients in the monitoring unit to smell a stick with a scent such as mint, fish, or banana. Electrodes in their brains showed patterns of neural activity "in areas we expected and areas we didn't expect," Richardson said. To better understand the brain's response, his team has just begun another round of experiments using a tool called an olfactometer, which will release more precisely timed bursts of odor.
Once researchers know where the brain becomes active in response to the smell of peppermint, they can try electrically stimulating those areas individually in hopes of producing the same sensation. "Compared to current technology, I think we're closer to inducing [odor perception] through brain stimulation than through olfactory bulb stimulation," Richardson said. He noted that there are already approved implants for brain stimulation and said using such devices would make the regulatory path easier. However, the distributed nature of odor perception within the brain introduces a new complication: users may need multiple implants to stimulate different areas. "We may need to visit different sites in quick succession or simultaneously," he said.
Road to Commercialization
In Europe, the European Union is funding its own olfactory implant project called ROSE (Restoring Odor Detection and Identification in Odor Deficiency). It launches in 2021 and involves seven institutions across Europe.
Thomas Hummel, head of the Smell and Taste Clinic at TU Dresden and a member of the consortium, said ROSE researchers are working with the French company Aryballe, which makes microsensors for odor analysis. The partners are currently experimenting with stimulating the olfactory bulb and prefrontal cortex. “All the components needed for the equipment are already there,” he said. "The difficulty is bringing them together." Hummel estimates that the consortium's research could lead to a commercial product within five to 10 years. "It's a matter of effort and funding," he said.
The jury is still out on whether neuroprostheses will be commercially viable, said electronic nose expert Persaud. "Some people with anosmia will do whatever it takes to have that sense restored to them," he said. "The question is whether there are enough people to create a market for this device," he said, because there is always some risk associated with surgery and implants.
VCU researchers have held informal meetings with regulators at the U.S. Food and Drug Administration, who have begun the early steps toward approving the implantable medical device. But Moorhead, an investor who tends to focus on practical issues, said the dream team likely won't take the technology all the way to the finish line of an FDA-approved commercial system. He noted that many existing medical implant companies have this expertise, such as Australian company Cochlear, which dominates the cochlear implant market. "If I can get [the project] to a stage where it's attractive to one of the companies, if I can take on some risk for them, that would be my best effort," Moorehead said.
Restoring people’s ability to smell and taste is the ultimate goal, Costanzo said. But before that, he could give them something else. He often gets calls from desperate people who have seen his work. “They’re very grateful that someone is working on a solution,” Costanzo said. "My goal is to bring hope to these people."
Source:
https://spectrum.ieee.org/covid-smell-prosthetic
The above is the detailed content of With this 'bionic nose', COVID-19 survivors will have the opportunity to 'smell the roses' again. For more information, please follow other related articles on the PHP Chinese website!

Hot AI Tools

Undresser.AI Undress
AI-powered app for creating realistic nude photos

AI Clothes Remover
Online AI tool for removing clothes from photos.

Undress AI Tool
Undress images for free

Clothoff.io
AI clothes remover

AI Hentai Generator
Generate AI Hentai for free.

Hot Article

Hot Tools

Notepad++7.3.1
Easy-to-use and free code editor

SublimeText3 Chinese version
Chinese version, very easy to use

Zend Studio 13.0.1
Powerful PHP integrated development environment

Dreamweaver CS6
Visual web development tools

SublimeText3 Mac version
God-level code editing software (SublimeText3)

Hot Topics
 1378
1378
 52
52
 I Tried Vibe Coding with Cursor AI and It's Amazing!
Mar 20, 2025 pm 03:34 PM
I Tried Vibe Coding with Cursor AI and It's Amazing!
Mar 20, 2025 pm 03:34 PM
Vibe coding is reshaping the world of software development by letting us create applications using natural language instead of endless lines of code. Inspired by visionaries like Andrej Karpathy, this innovative approach lets dev
 Top 5 GenAI Launches of February 2025: GPT-4.5, Grok-3 & More!
Mar 22, 2025 am 10:58 AM
Top 5 GenAI Launches of February 2025: GPT-4.5, Grok-3 & More!
Mar 22, 2025 am 10:58 AM
February 2025 has been yet another game-changing month for generative AI, bringing us some of the most anticipated model upgrades and groundbreaking new features. From xAI’s Grok 3 and Anthropic’s Claude 3.7 Sonnet, to OpenAI’s G
 How to Use YOLO v12 for Object Detection?
Mar 22, 2025 am 11:07 AM
How to Use YOLO v12 for Object Detection?
Mar 22, 2025 am 11:07 AM
YOLO (You Only Look Once) has been a leading real-time object detection framework, with each iteration improving upon the previous versions. The latest version YOLO v12 introduces advancements that significantly enhance accuracy
 Is ChatGPT 4 O available?
Mar 28, 2025 pm 05:29 PM
Is ChatGPT 4 O available?
Mar 28, 2025 pm 05:29 PM
ChatGPT 4 is currently available and widely used, demonstrating significant improvements in understanding context and generating coherent responses compared to its predecessors like ChatGPT 3.5. Future developments may include more personalized interactions and real-time data processing capabilities, further enhancing its potential for various applications.
 Best AI Art Generators (Free & Paid) for Creative Projects
Apr 02, 2025 pm 06:10 PM
Best AI Art Generators (Free & Paid) for Creative Projects
Apr 02, 2025 pm 06:10 PM
The article reviews top AI art generators, discussing their features, suitability for creative projects, and value. It highlights Midjourney as the best value for professionals and recommends DALL-E 2 for high-quality, customizable art.
 o1 vs GPT-4o: Is OpenAI's New Model Better Than GPT-4o?
Mar 16, 2025 am 11:47 AM
o1 vs GPT-4o: Is OpenAI's New Model Better Than GPT-4o?
Mar 16, 2025 am 11:47 AM
OpenAI's o1: A 12-Day Gift Spree Begins with Their Most Powerful Model Yet December's arrival brings a global slowdown, snowflakes in some parts of the world, but OpenAI is just getting started. Sam Altman and his team are launching a 12-day gift ex
 Google's GenCast: Weather Forecasting With GenCast Mini Demo
Mar 16, 2025 pm 01:46 PM
Google's GenCast: Weather Forecasting With GenCast Mini Demo
Mar 16, 2025 pm 01:46 PM
Google DeepMind's GenCast: A Revolutionary AI for Weather Forecasting Weather forecasting has undergone a dramatic transformation, moving from rudimentary observations to sophisticated AI-powered predictions. Google DeepMind's GenCast, a groundbreak
 Which AI is better than ChatGPT?
Mar 18, 2025 pm 06:05 PM
Which AI is better than ChatGPT?
Mar 18, 2025 pm 06:05 PM
The article discusses AI models surpassing ChatGPT, like LaMDA, LLaMA, and Grok, highlighting their advantages in accuracy, understanding, and industry impact.(159 characters)