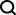Technology peripherals
Technology peripherals
 AI
AI
 Plug in the electrodes and Musk will cut your brain! Neuralink clinical trial approved by FDA, brain-computer interface project reaches new milestone
Plug in the electrodes and Musk will cut your brain! Neuralink clinical trial approved by FDA, brain-computer interface project reaches new milestone
Plug in the electrodes and Musk will cut your brain! Neuralink clinical trial approved by FDA, brain-computer interface project reaches new milestone
Musk’s brain-computer interface dream has reached a new milestone.
Just now, Neuralink shockedly announced that the first human clinical study of a brain-computer interface experiment has been approved by the U.S. Food and Drug Administration (FDA).
Guoke.com CEO Ji Shisan commented excitedly: This is a big step in the field of brain-computer interface! Carbon-based silicon-based fusion is promising, and may be realized within 5-10 years.
It is reported that clinical trials are not yet open for recruitment, but it should not be long.
Where are the students who intend to become "carbon-based silicon-based" life forms? Please raise your hands!
Academician Ma’s Difficult Quest
This wave of Neuralink’s FDA approval can be said to have marked a milestone for the brain-computer interface industry.
Neuralink was founded in 2016 and has attracted some of the top neuroscientists to join.
Neuralink has been working on developing a small device that connects the human brain and computers.
This device consists of electrode wires, and placement of the device requires drilling into the skull.
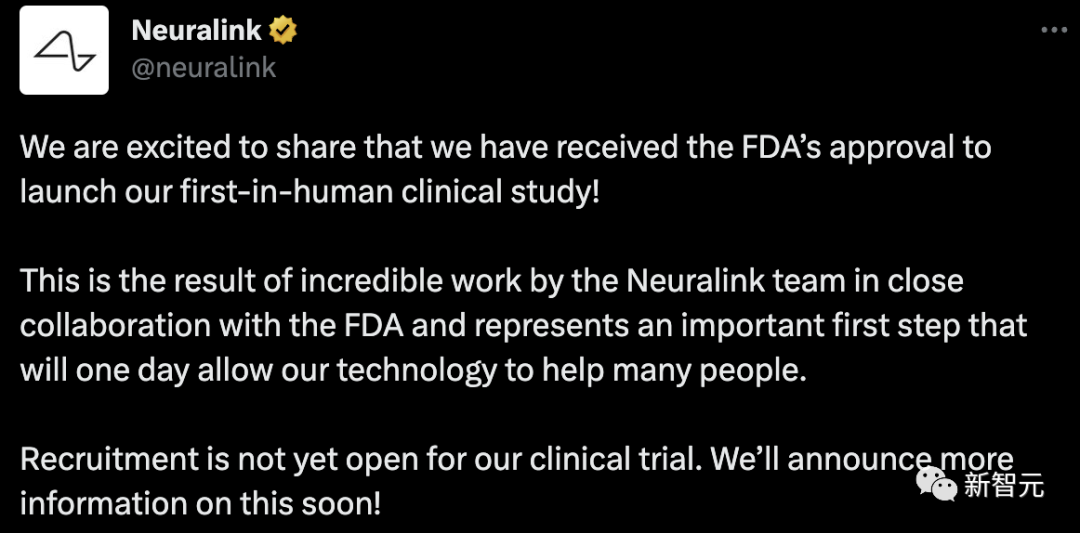
Neuralink’s goal is to use this device to help people with paralysis or traumatic brain injuries to communicate and control computers through their thoughts alone.
And Musk’s ultimate goal is to use brain-computer interfaces to allow humans to keep up with AI.
Since the beginning of 2019, crazy Musk has been promoting it everywhere.

However, Musk also knows that he cannot fight an unprepared battle. Neuralink was established in 2016, and it was not until 2022 that it applied to the FDA for approval to conduct human experiments on brain-computer interfaces.
However, the FDA at that time still had a lot of worries about this technology and rejected Musk, fearing that Xiaoma would not cure the disease but would cause problems for people.
The FDA's rejection is justified, mainly due to "major safety issues."
Because the brain is very, very soft and very fragile, migrating wires can induce inflammation, impair function in critical areas of the brain and rupture blood vessels.
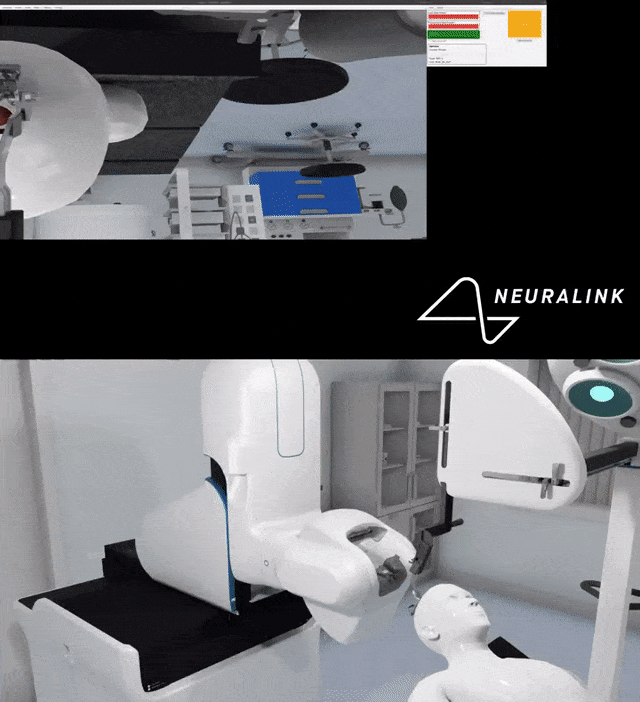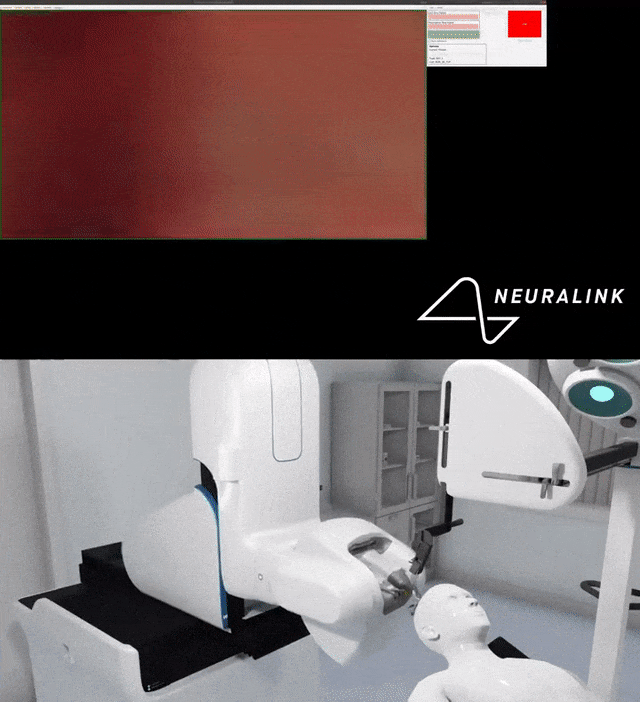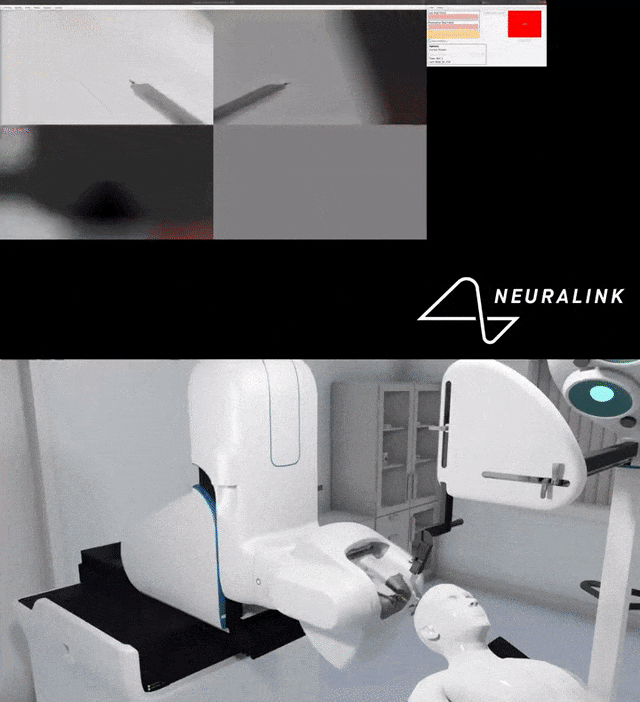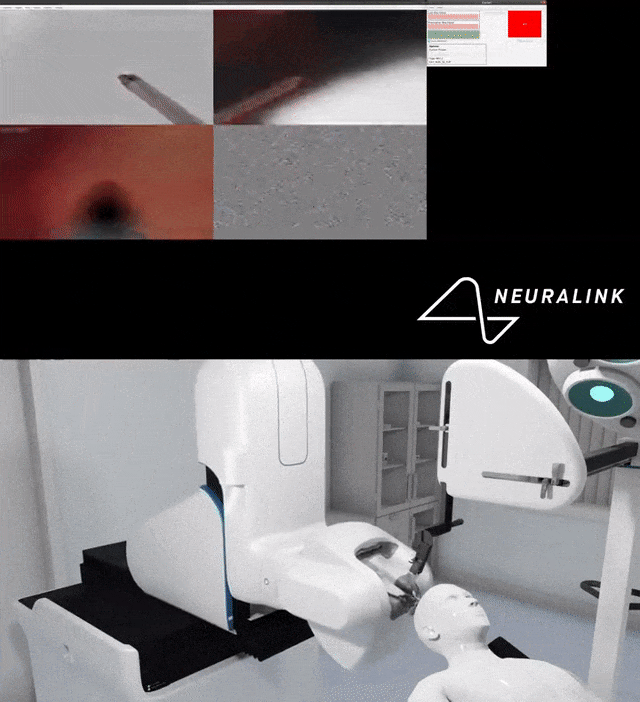
Multiple small parts and wires can become displaced in the brain, which can cause damage.
If any component of the device connected to the battery current fails, the current can also damage brain tissue.
There are even issues with the device overheating, and these bugs and issues need to be overcome one by one before testing is allowed.
## Such technological breakthroughs represent potential hope for anyone suffering from debilitating diseases. But putting a foreign object, let alone a large amount of it, into the brain is fundamentally dangerous.
Having said that, the American technology company Synchron is one step ahead of Neuralink in its experiments on brain-computer interfaces.
This company received FDA approval as early as 2021 and completed the "first" brain-computer interface surgery in the United States in July last year.
Now, Synchron has recruited its first patient for clinical trials. Synchron's device uses different technology than Neuralink's device and is less invasive.
However, although the FDA has approved it, Neuralink said it has not yet opened clinical trials.
In the tweet, Neuralink also stated that this approval is a milestone step and will be able to use this technology to help many people in the future.
Musk also put aside his rhetoric, saying that he is very confident in the safety of Neuralink's equipment and is willing to implant it in his children's bodies.
He imagines that surgery or implantable devices could become more accessible for both disabled and able-bodied people. These devices can treat a wide range of conditions, including obesity, autism, depression and schizophrenia, among many others.
Who is the first ghost
What does this mean?
From now on, Neuralink will implant devices into the heads of living people.
In November last year, Musk said at the press conference: There are still about six months to go before Neuralink’s first human experiment.
I’m pretty sure that if the iPhone 14 is available, you won’t want the iPhone 1 to remain in your mind.

His promise rarely came true.
Let us imagine, who will sign up for clinical trial recruitment and become the first volunteer for the brain-computer interface experiment?
Maybe it’s someone who wants to get medical treatment; maybe it’s someone who wants to stand next to Musk in the spotlight and become famous; maybe it’s Musk himself ?
After all, he once publicly stated that he would definitely implant brain-computer equipment into his brain at some point in the future.
If you can't contain your excitement and want to sign up for clinical trials now, I'm afraid you have to wait for news from Neuralink.
Finally breathed a sigh of relief
No matter what, this news undoubtedly relieved Academician Ma and his Neraulink. After all, there has been a long-standing dispute between the company and the regulatory agencies. Mostly negative news.
Previously, Neraulink not only had its clinical trial application rejected by the FDA, but was also accused of mistreating research animals and violating regulations by transporting contaminated implants containing monkey tissue and pathogens. Transportation Management Regulations.
At the time, Neuralink denied this statement.
They responded to a federal complaint from the nonprofit Physicians Committee for Responsible Medicine (PCRM) and stated that our lab does meet, and has always met, federally mandated standards.
Normally, when the FDA rejects a clinical trial application, it is willing to explain in detail why it believes the trial plan is inadequate.
Neuralink's ability to address the concerns of federal regulators in a relatively short period of time is a positive sign for the company.
Now, the main obstacle has been cleared.
Recently, Neuralink has not been idle, constantly updating some progress on brain-computer interfaces on Twitter.
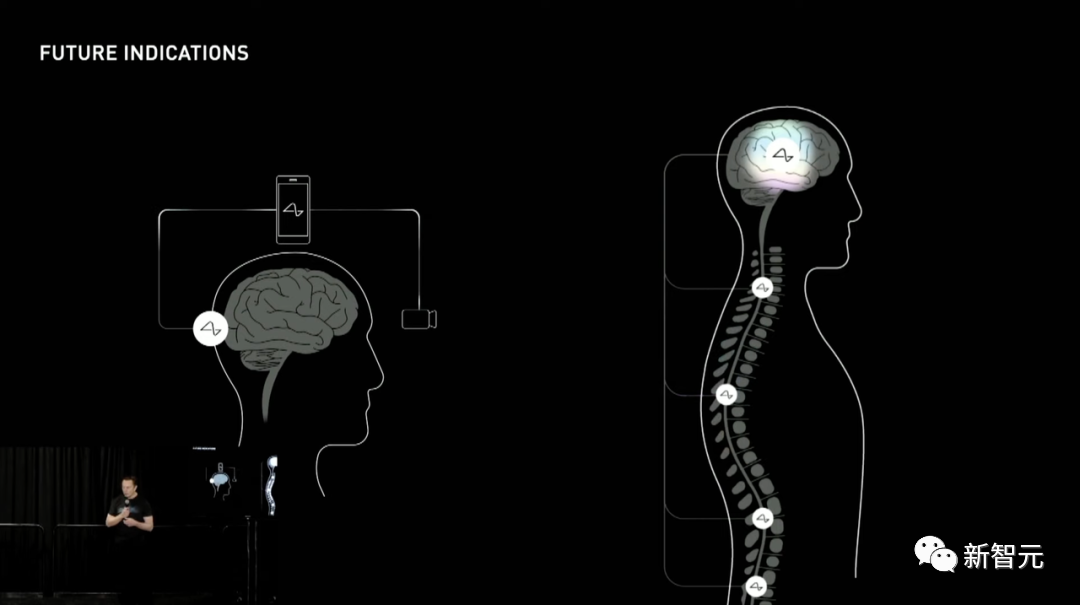
For example, Neuralink’s surgical robot uses an advanced imaging system to examine the brain and insert electrode wires away from blood vessels.

At the same time, the staff also tested the accuracy of each high-precision camera of the surgical robot.
You can see the detailed process done by the robot.
Use a dynamic mechanical analyzer to fatigue test electrode wires and identify changes in their mechanical properties over time.
To ensure safety and improve efficiency, Neuralink also tested the thermal performance of the implant.
Below are infrared images taken to check that the bottom surface of the implant remains uncool while the charging coil is being charged at different locations.
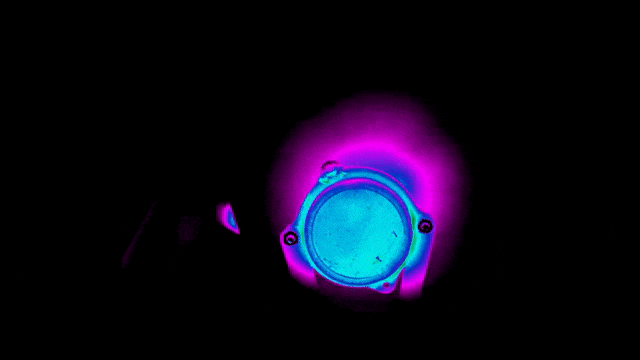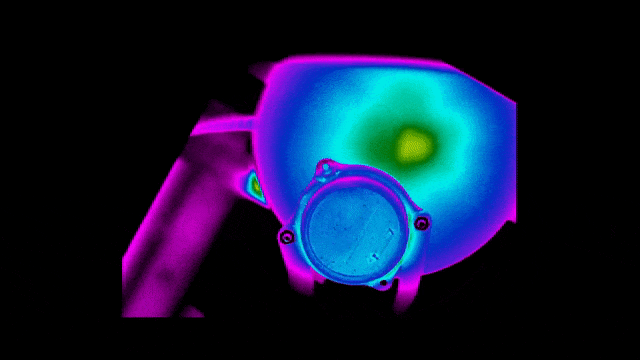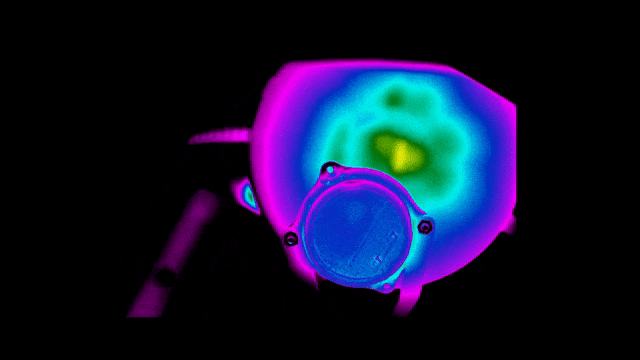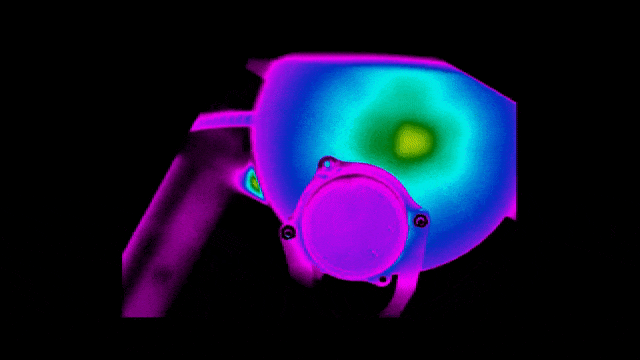
Of course, practice brings true knowledge.
The Neuralink team works together to conduct simulation experiments to quickly test and improve the surgery.
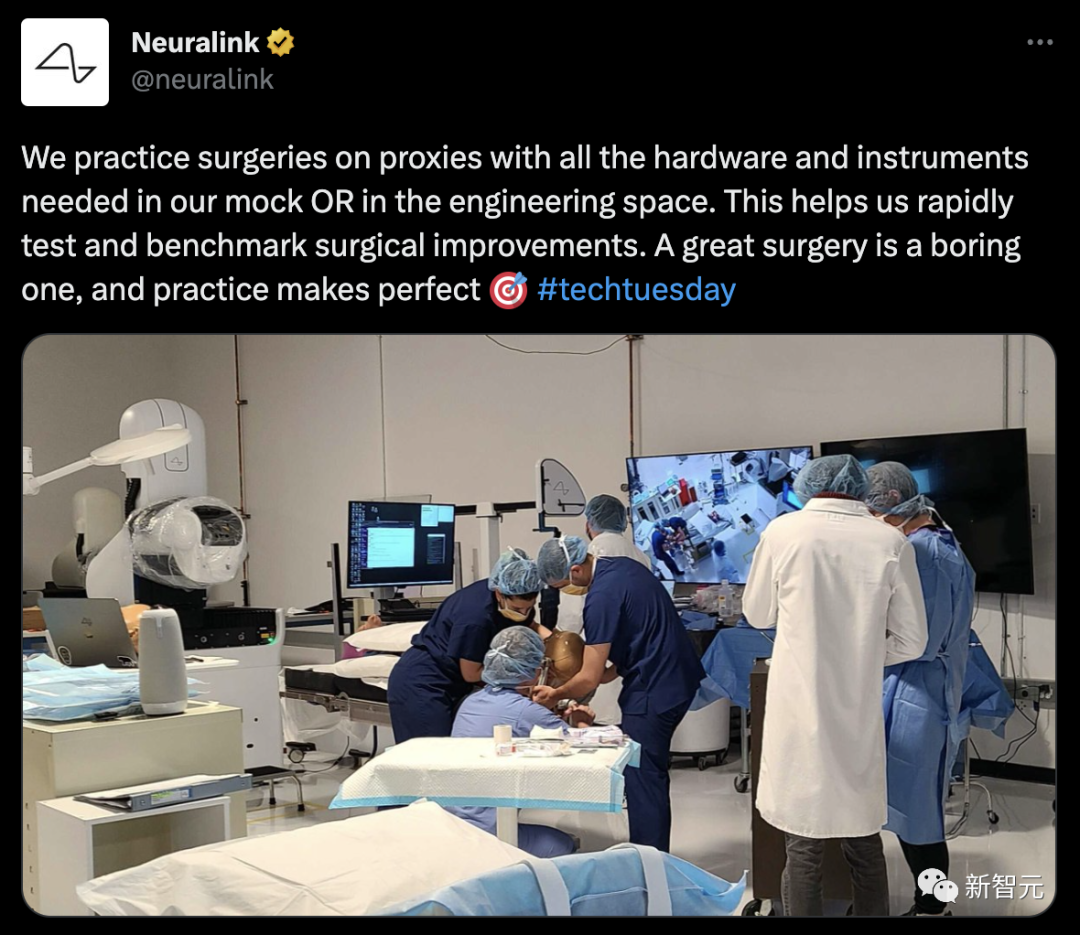
Despite FDA approval, the large-scale use of brain-computer interfaces is still far away.
Cristin Welle, a former FDA official and associate professor of neurosurgery and physiology at the University of Colorado, said Neuralink's device will be at least five to 10 years away from commercialization.
Previously, it took Synchron nearly a year from FDA approval to actual implantation.
Netizen: Let me be the first
Some netizens have volunteered to be the first person to be "intubated" by Neuralink.
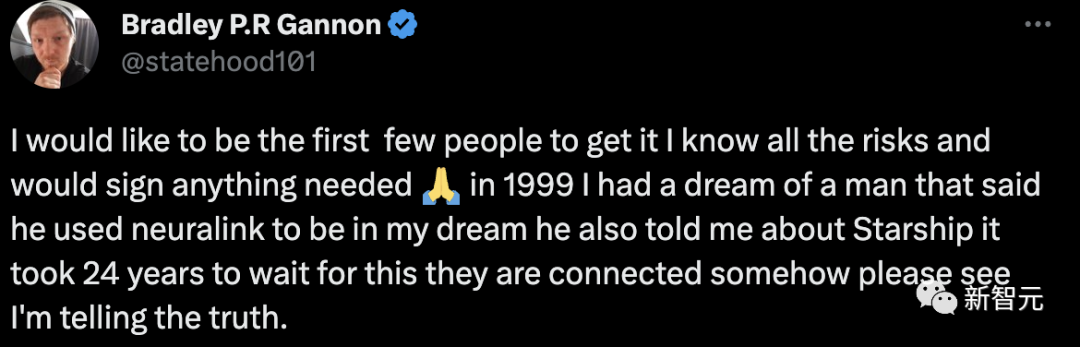
"I want to be one of the first to experiment, I know all the risks and am willing to sign any necessary documents."
In 1999, I had a dream. The person in the dream told me that he used Neuralink to appear in my dream. He also introduced me to Starship. I have been waiting for this to come true for 24 years. Between them, Connected in some way, please bear witness that what I said is true.
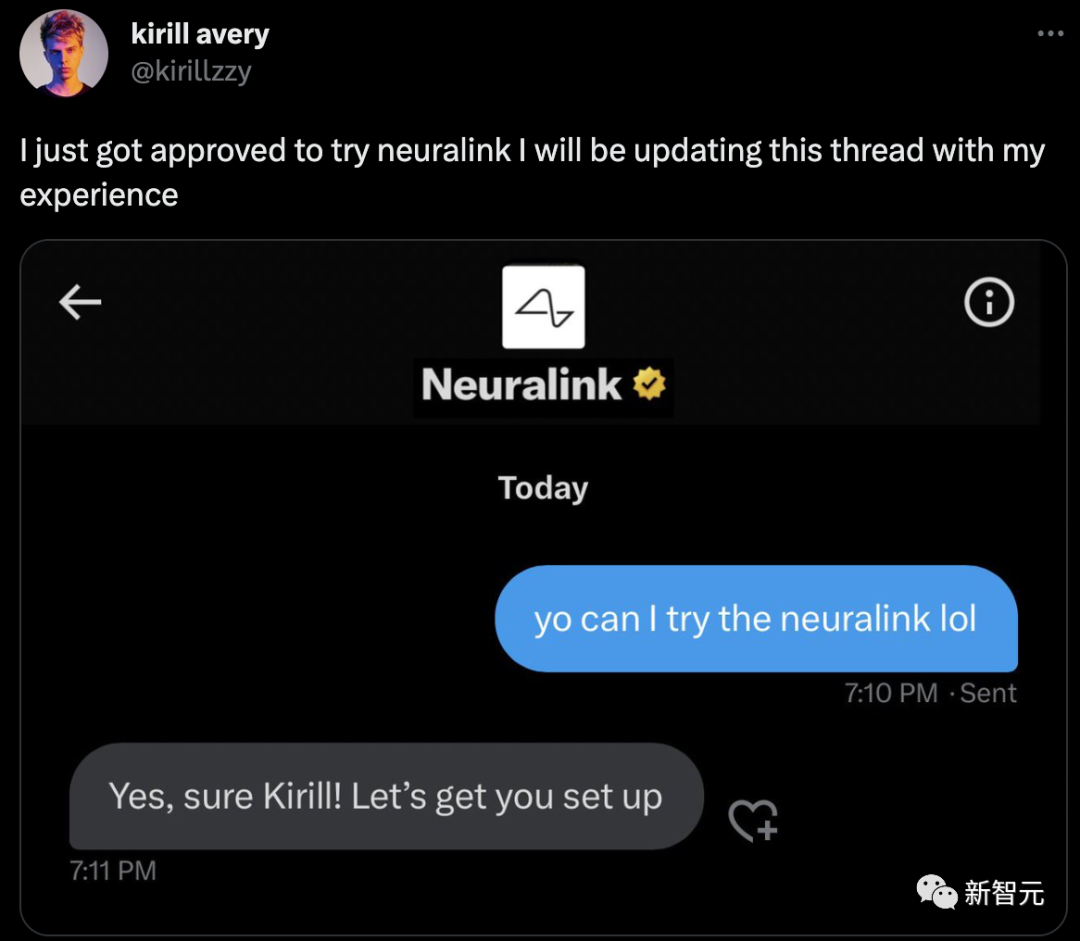
There are still people lined up.
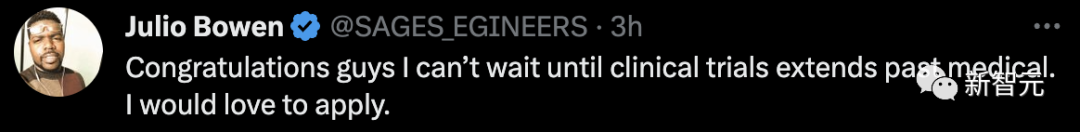
I can’t wait until clinical trials extend into the medical field. I would love to apply.
Stop talking nonsense and connect to me quickly.
The next new era of human-computer interaction is here.
The above is the detailed content of Plug in the electrodes and Musk will cut your brain! Neuralink clinical trial approved by FDA, brain-computer interface project reaches new milestone. For more information, please follow other related articles on the PHP Chinese website!

Hot AI Tools
Undresser.AI Undress
AI-powered app for creating realistic nude photos
AI Clothes Remover
Online AI tool for removing clothes from photos.

Undress AI Tool
Undress images for free

Clothoff.io
AI clothes remover
Video Face Swap
Swap faces in any video effortlessly with our completely free AI face swap tool!

Hot Article

Hot Tools

Notepad++7.3.1
Easy-to-use and free code editor

SublimeText3 Chinese version
Chinese version, very easy to use

Zend Studio 13.0.1
Powerful PHP integrated development environment

Dreamweaver CS6
Visual web development tools

SublimeText3 Mac version
God-level code editing software (SublimeText3)

Hot Topics
 1387
1387
 52
52
 How to get logged in user information in WordPress for personalized results
Apr 19, 2025 pm 11:57 PM
How to get logged in user information in WordPress for personalized results
Apr 19, 2025 pm 11:57 PM
Recently, we showed you how to create a personalized experience for users by allowing users to save their favorite posts in a personalized library. You can take personalized results to another level by using their names in some places (i.e., welcome screens). Fortunately, WordPress makes it very easy to get information about logged in users. In this article, we will show you how to retrieve information related to the currently logged in user. We will use the get_currentuserinfo(); function. This can be used anywhere in the theme (header, footer, sidebar, page template, etc.). In order for it to work, the user must be logged in. So we need to use
 How to elegantly obtain entity class variable names to build database query conditions?
Apr 19, 2025 pm 11:42 PM
How to elegantly obtain entity class variable names to build database query conditions?
Apr 19, 2025 pm 11:42 PM
When using MyBatis-Plus or other ORM frameworks for database operations, it is often necessary to construct query conditions based on the attribute name of the entity class. If you manually every time...
 Java BigDecimal operation: How to accurately control the accuracy of calculation results?
Apr 19, 2025 pm 11:39 PM
Java BigDecimal operation: How to accurately control the accuracy of calculation results?
Apr 19, 2025 pm 11:39 PM
Java...
 How to process and display percentage numbers in Java?
Apr 19, 2025 pm 10:48 PM
How to process and display percentage numbers in Java?
Apr 19, 2025 pm 10:48 PM
Display and processing of percentage numbers in Java In Java programming, the need to process and display percentage numbers is very common, for example, when processing Excel tables...
 How to package in IntelliJ IDEA for specific Git versions to avoid including unfinished code?
Apr 19, 2025 pm 08:18 PM
How to package in IntelliJ IDEA for specific Git versions to avoid including unfinished code?
Apr 19, 2025 pm 08:18 PM
In IntelliJ...
 How do subclasses modify private properties by inheriting the public method of parent class?
Apr 19, 2025 pm 11:12 PM
How do subclasses modify private properties by inheriting the public method of parent class?
Apr 19, 2025 pm 11:12 PM
How to modify private properties by inheriting the parent class's public method When learning object-oriented programming, understanding the inheritance of a class and access to private properties is a...
 How to efficiently query large amounts of personnel data through natural language processing?
Apr 19, 2025 pm 09:45 PM
How to efficiently query large amounts of personnel data through natural language processing?
Apr 19, 2025 pm 09:45 PM
Effective method of querying personnel data through natural language processing How to efficiently use natural language processing (NLP) technology when processing large amounts of personnel data...
 What are the underlying principles and lock upgrade process of synchronized in Java?
Apr 19, 2025 pm 09:48 PM
What are the underlying principles and lock upgrade process of synchronized in Java?
Apr 19, 2025 pm 09:48 PM
Exploring the underlying principle of synchronized and the details of the lock upgrade process In Java, synchronized keyword is one of the tools used to implement thread synchronization, and its underlying...