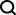Technology peripherals
Technology peripherals
 AI
AI
 World's first: Molecular Heart's new AI algorithm to overcome the problems of protein side chain prediction and sequence design
World's first: Molecular Heart's new AI algorithm to overcome the problems of protein side chain prediction and sequence design
World's first: Molecular Heart's new AI algorithm to overcome the problems of protein side chain prediction and sequence design
Heart of Machine Column
Heart of Machine Editorial Department
PSCP deep architecture AttnPacker - greatly optimized AI algorithm.
The formation of protein structure and function depends largely on the interaction between side chain atoms. Therefore, accurate protein side chain prediction (PSCP) is a key link in solving the problems of protein structure prediction and protein design. However, previous protein structure predictions mostly focused on the main chain structure, and side chain structure prediction has always been a difficult problem that has not been completely solved.
Recently, Xu Jinbo’s team at Molecular Heart launched a new PSCP deep architecture AttnPacker, which has achieved significant improvements in speed, memory efficiency and overall accuracy. It is currently the best known side-chain structure prediction algorithm and the first in the world. An AI algorithm that can perform protein side chain prediction and sequence design at the same time.
The paper was published in the Proceedings of the National Academy of Sciences (PNAS), and its pre-training model, source code and inference scripts have been open sourced on Github.

Paper link:
https://www.pnas.org/doi/10.1073/pnas.2216438120#supplementary-materials
Open source link:
https://github.com/MattMcPartlon/AttnPacker
background
Protein is folded from several amino acids, and its structure is divided into main chain and side chain. Differences in side chains have a huge impact on protein structure and function, especially biological activity. Based on a clear understanding of the side chain structure, scientists can more accurately determine the three-dimensional structure of proteins, analyze protein-protein interactions, and conduct rational protein design. When applied to the field of drug design, scientists can quickly and more accurately find suitable binding sites for drugs and receptors, and even optimize or design binding sites as needed; in the field of enzyme optimization, scientists can optimize sequences Transformation allows multiple side chains to participate in catalytic reactions to achieve more efficient and specific catalytic effects.
Most of the current protein structure prediction algorithms mainly focus on the structural analysis of the main chain, but protein side chain structure prediction is still a problem that has not been completely overcome. Whether it is popular protein structure prediction algorithms such as AlphaFold2 or algorithms focusing on side chain structure prediction such as DLPacker and RosettaPacker, the accuracy or speed are not satisfactory. This also imposes limitations on protein design.
Traditional methods, such as RosettaPacker, mainly use energy optimization methods, first grouping the distribution of side chain atoms, and then searching the grouping of side chains for a specific amino acid to find the combination with the smallest energy. These methods differ primarily from the researcher's choice of rotamer libraries, energy functions, and energy minimization procedures, with accuracy limited by the use of search heuristics and discrete sampling procedures. There are also side chain prediction methods based on deep learning in the industry, such as DLPacker, which formulates PSCP as an image-to-image conversion problem and adopts a U-net model structure. However, the prediction accuracy and speed are still not ideal.
method
AttnPacker is an end-to-end deep learning method for predicting protein side chain coordinates. It jointly simulates side chain interactions, with directly predicted side chain structures that are more physically feasible, with fewer atomic collisions and more ideal bond lengths and angles.
Specifically, AttnPacker introduces a depth map converter architecture that leverages the geometric and relational aspects of PSCP. Inspired by AlphaFold2, Molecular Heart proposes position-aware triangle updates to optimize pairwise features using a graph-based framework to compute triangle attention and multiplicative updates. With this approach, AttnPacker has significantly less memory and a higher capacity model. Furthermore, Molecular Heart explores several SE (3) equivariant attention mechanisms and proposes an equivariant transformer architecture for learning from 3D points.
AttnPacker runs the process. The protein backbone coordinates and sequence are used as input, and the spatial feature map and equivariable basis are derived based on the coordinate information. The feature map is processed by the invariant graph-transformer module and then passed to an equivariant TFN-Transformer that outputs predicted side chain coordinates, confidence scores for each residue, and optional design sequences. The predicted coordinates are post-processed to remove all spatial conflicts and ensure idealized geometry.
Effect
In terms of prediction performance, AttnPacker shows improvements in accuracy and efficiency for both natural and non-natural backbone structures. At the same time, physical feasibility is ensured, deviations from ideal bond lengths and angles are negligible, and minimal atomic steric hindrance is produced.
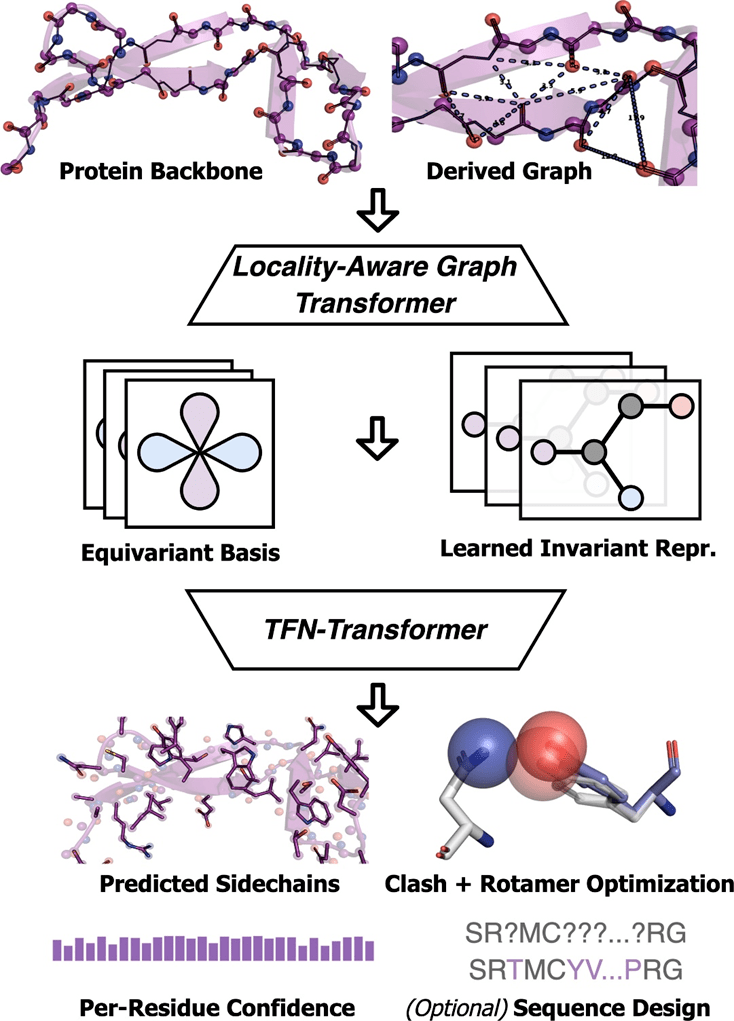
Molecular Heart conducted a comparative test on AttnPacker with the current state-of-the-art methods - SCWRL4, FASPR, RosettaPacker and DLPacker on the CASP13 and CASP14 natural and non-native protein backbone data sets. Results show that AttnPacker significantly outperforms traditional protein side chain prediction methods on CASP13 and CASP14 native backbones, with average reconstruction RMSDs more than 18% lower than the suboptimal method on each test set. AttnPacker also outperforms the deep learning method DLPacker, reducing average RMSD by more than 11% while also significantly improving sidechain dihedral accuracy. In addition to accuracy, AttnPacker has significantly fewer atomic collisions than other methods.
Given the natural main chain structure, the side chain structure prediction results of each algorithm on the CASP13 and CASP14 target proteins are given. Asterisks indicate that the average conflict values are lower than the native structure—56.0, 5.9, and 0.4 for CASP13 and 80.4, 7.9, and 2.5 for CASP14.
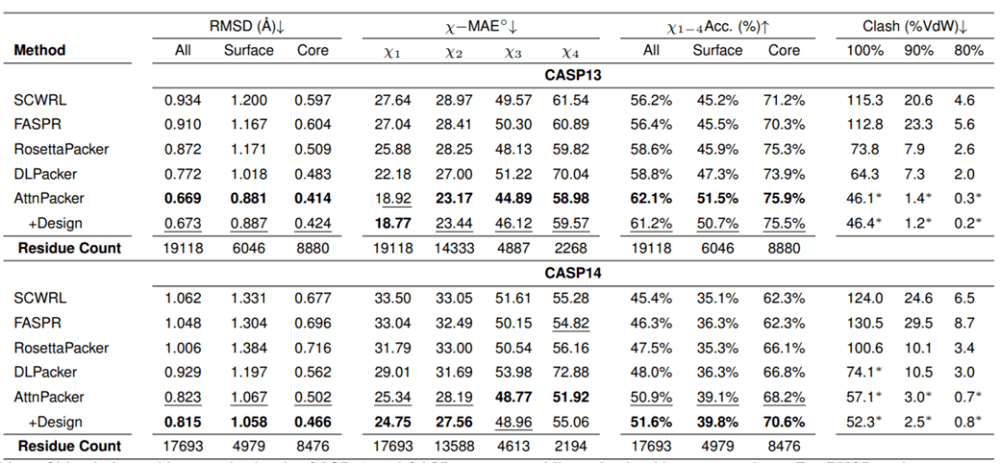
On CASP13 and CASP14 non-native backbones, AttnPacker is also significantly better than other methods, and atomic collisions are also significantly less than other methods.
Given the non-natural backbone structure, the side chain structure prediction results of each algorithm on the CASP13 and CASP14 target proteins are given. Asterisks indicate that the average conflict values are lower than the corresponding native structures—34.6, 2.2, 0.5 for CASP13 and 40.0, 2.7, 0.7 for CASP14.
Innovatively abandons the discrete rotamer library and computationally expensive conformational search and sampling steps, and directly combines the main chain 3D geometric structure to calculate all side chain coordinates in parallel. Compared with the deep learning-based method DLPacker and the traditional computing method-based RosettaPacker, AttnPacker has significantly improved computing efficiency and reduced inference time by more than 100 times.

Time comparison of different PSCP methods. Reconstructing the relative times of side chain atoms for all 83 CASP13 target proteins.
AttnPacker performs equally well in protein design. Molecular Heart trained an AttnPacker variant for co-design that achieves native sequence recovery rates comparable to current state-of-the-art methods while also producing highly accurate assemblies. Rosetta simulation validation shows that AttnPacker-designed structures generally produce subnative (lower) Rosetta energies.
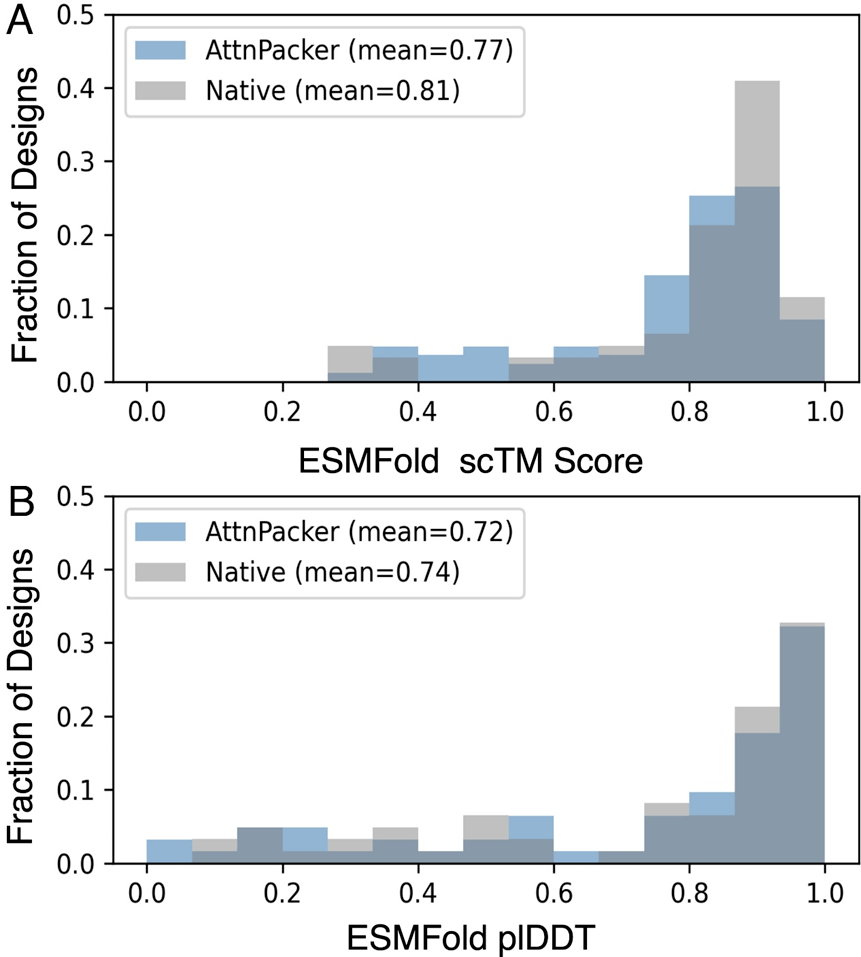
The ESMFold scTM and plDDT indicators were used to compare the native protein sequence and the sequence generated by AttnPacker to evaluate the quality of AttnPacker's generation, and the results showed a strong correlation.
In addition to its amazing effects and efficiency, AttnPaker also has a very practical value - it is very easy to use. AttnPaker only requires a protein structure file to run. In contrast, OPUS-Rota4 (28) requires a voxel representation of the atomic environment from DLPacker, logic, secondary structure from trRosetta100, and constraint files from OPUS-CM output. Additionally, since AttnPacker directly predicts side chain coordinates, the output is fully differentiable, which facilitates downstream prediction tasks such as optimization or protein-protein interactions. "The advantages of good prediction effect, high efficiency and ease of use are conducive to the widespread use of AttnPacker in research and industrial fields." Professor Xu Jinbo said.
Summarize
1. AttnPacker is a SE (3) equivariant model used to directly predict sequence and side chain coordinates. It can be used for protein side chain structure prediction and protein sequence design. It is a pioneering work.
2. AttnPacker's accuracy is better than other methods, its efficiency is greatly improved, and it is extremely easy to use.
The above is the detailed content of World's first: Molecular Heart's new AI algorithm to overcome the problems of protein side chain prediction and sequence design. For more information, please follow other related articles on the PHP Chinese website!

Hot AI Tools

Undresser.AI Undress
AI-powered app for creating realistic nude photos

AI Clothes Remover
Online AI tool for removing clothes from photos.

Undress AI Tool
Undress images for free

Clothoff.io
AI clothes remover

AI Hentai Generator
Generate AI Hentai for free.

Hot Article

Hot Tools

Notepad++7.3.1
Easy-to-use and free code editor

SublimeText3 Chinese version
Chinese version, very easy to use

Zend Studio 13.0.1
Powerful PHP integrated development environment

Dreamweaver CS6
Visual web development tools

SublimeText3 Mac version
God-level code editing software (SublimeText3)

Hot Topics
 1386
1386
 52
52
 I Tried Vibe Coding with Cursor AI and It's Amazing!
Mar 20, 2025 pm 03:34 PM
I Tried Vibe Coding with Cursor AI and It's Amazing!
Mar 20, 2025 pm 03:34 PM
Vibe coding is reshaping the world of software development by letting us create applications using natural language instead of endless lines of code. Inspired by visionaries like Andrej Karpathy, this innovative approach lets dev
 Top 5 GenAI Launches of February 2025: GPT-4.5, Grok-3 & More!
Mar 22, 2025 am 10:58 AM
Top 5 GenAI Launches of February 2025: GPT-4.5, Grok-3 & More!
Mar 22, 2025 am 10:58 AM
February 2025 has been yet another game-changing month for generative AI, bringing us some of the most anticipated model upgrades and groundbreaking new features. From xAI’s Grok 3 and Anthropic’s Claude 3.7 Sonnet, to OpenAI’s G
 How to Use YOLO v12 for Object Detection?
Mar 22, 2025 am 11:07 AM
How to Use YOLO v12 for Object Detection?
Mar 22, 2025 am 11:07 AM
YOLO (You Only Look Once) has been a leading real-time object detection framework, with each iteration improving upon the previous versions. The latest version YOLO v12 introduces advancements that significantly enhance accuracy
 Best AI Art Generators (Free & Paid) for Creative Projects
Apr 02, 2025 pm 06:10 PM
Best AI Art Generators (Free & Paid) for Creative Projects
Apr 02, 2025 pm 06:10 PM
The article reviews top AI art generators, discussing their features, suitability for creative projects, and value. It highlights Midjourney as the best value for professionals and recommends DALL-E 2 for high-quality, customizable art.
 Is ChatGPT 4 O available?
Mar 28, 2025 pm 05:29 PM
Is ChatGPT 4 O available?
Mar 28, 2025 pm 05:29 PM
ChatGPT 4 is currently available and widely used, demonstrating significant improvements in understanding context and generating coherent responses compared to its predecessors like ChatGPT 3.5. Future developments may include more personalized interactions and real-time data processing capabilities, further enhancing its potential for various applications.
 Best AI Chatbots Compared (ChatGPT, Gemini, Claude & More)
Apr 02, 2025 pm 06:09 PM
Best AI Chatbots Compared (ChatGPT, Gemini, Claude & More)
Apr 02, 2025 pm 06:09 PM
The article compares top AI chatbots like ChatGPT, Gemini, and Claude, focusing on their unique features, customization options, and performance in natural language processing and reliability.
 How to Use Mistral OCR for Your Next RAG Model
Mar 21, 2025 am 11:11 AM
How to Use Mistral OCR for Your Next RAG Model
Mar 21, 2025 am 11:11 AM
Mistral OCR: Revolutionizing Retrieval-Augmented Generation with Multimodal Document Understanding Retrieval-Augmented Generation (RAG) systems have significantly advanced AI capabilities, enabling access to vast data stores for more informed respons
 Top AI Writing Assistants to Boost Your Content Creation
Apr 02, 2025 pm 06:11 PM
Top AI Writing Assistants to Boost Your Content Creation
Apr 02, 2025 pm 06:11 PM
The article discusses top AI writing assistants like Grammarly, Jasper, Copy.ai, Writesonic, and Rytr, focusing on their unique features for content creation. It argues that Jasper excels in SEO optimization, while AI tools help maintain tone consist