
According to news on June 9, AVATAR MEDICAL, a VR medical imaging solution provider for the surgical field, announced that its VR preoperative planning solution has obtained FDA 510(k) certification and is expected to obtain European medical device certification next year. It is reported that 510(k) mainly certifies medical devices entering the US market. After approval, AVATAR MEDICAL’s VR solution will be more widely used in medical scenarios.
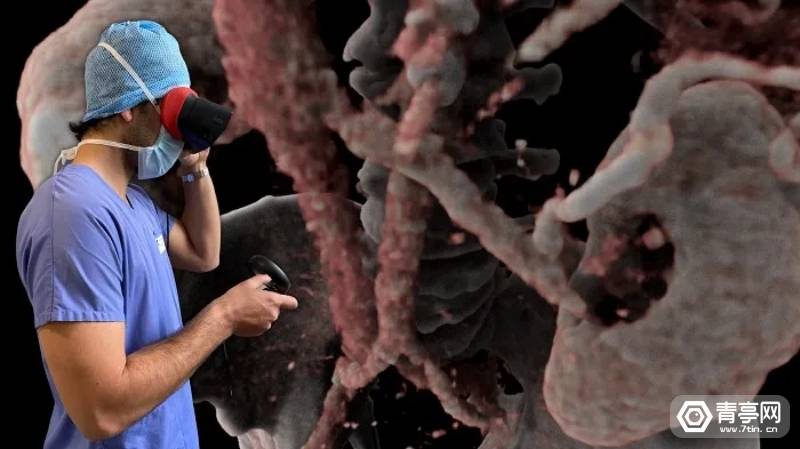
According to Qingting.com, AVATAR MEDICAL is committed to developing AR/VR medical visualization solutions, which are characterized by allowing surgeons to intuitively view 3D patient models generated based on MRI and CT from multiple angles, and clearly identify patients in immersive scenes. Muscles, blood vessels, tumors and other soft tissues in the body. In addition to preoperative planning, this program can be used for training, patient communication, case studies, and to guide surgeons during surgery.
In the past 3 years, more than 100 surgeons have used the AVATAR MEDICAL solution, from 20 different institutions including UMass, CUNY, and Columbia University.
The above is the detailed content of AVATAR MEDICAL's VR surgical visualization solution obtains FDA certification. For more information, please follow other related articles on the PHP Chinese website!




