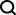Technology peripherals
Technology peripherals
 AI
AI
 With 130,000 annotated neurons and 53 million synapses, Princeton University and others released the first complete 'adult fly' brain connectome
With 130,000 annotated neurons and 53 million synapses, Princeton University and others released the first complete 'adult fly' brain connectome
With 130,000 annotated neurons and 53 million synapses, Princeton University and others released the first complete 'adult fly' brain connectome
As of today, there are many projects that have mapped the whole-brain connectome of various organisms, from Caenorhabditis elegans (302 neurons) to Drosophila melanogaster (~100,000 neurons). Drosophila melanogaster is one of the most thoroughly studied organisms by humans. As of 2017, eight Nobel Prizes have been awarded to research using fruit flies.
Researchers’ research on Drosophila continues. Recently, researchers from Princeton University and other institutions released the Drosophila whole-brain connectome, including about 130k annotated neurons. and tens of millions of types of synapses.

Everyone knows to some extent that a basic nervous system has existed since ancient animals, but the emergence of the brain system can be traced back to 500 million years ago . Research shows that dividing the brain into different regions helps understand its function.
However, for many years, there has been controversy about neural connection maps at the neuronal and synaptic level. The main reason for this phenomenon is that humans lack the ability to reconstruct such connection maps. technology. Things only started to change in the early 21st century as technology evolved.
Until today, researchers from Princeton University and other institutions have released the Drosophila whole-brain connectome, which is the first complete neural connection map of the adult Drosophila brain.
 Picture
Picture
Paper address: https://www.biorxiv.org/content/10.1101/2023.06.27.546656v1. full.pdf
 ##Picture
##Picture
After the release of the results, someone said: "Many projects have mapped the full range of various organisms. Map of the brain connectome, from Caenorhabditis elegans (302 neurons) to Drosophila melanogaster (~100k neurons). With our current computing power, why can't we perform accurate computer simulations of these organisms in a virtual 3D environment? 》
The first complete neural connection map of the adult fruit fly brain
The brain of fruit flies looks very small, with 10^5 Neurons and 10^8 synapses, nevertheless, fruit flies use these to see, smell, hear, walk, and of course fly.
For a long time, researchers have reconstructed parts of the Drosophila brain through electron microscope (EM) images. These images have good clarity and can show the small size of neurons. Branches and connected synapses. The resulting connectivity map of neural circuits provides key insights into how the brain generates social behaviors, memory-related behaviors, and navigation behaviors.
However, although the EM method has been applied to some areas of the brain and reconstructed information-rich local connection maps, this method is still insufficient for a more comprehensive understanding of brain function. insufficient.
Previously, researchers built a single synapse grid based on the research of Ito et al. The grid used in this article is based on the JFRC2 standard brain template that previously generated complete brain segmentation. These Grids are also used in the Virtual Fly Brain project. This study moves these meshes from JFRC2 space into FlyWire (FAFB14.1) space through a series of non-rigid transformations.
Note: FlyWire is a whole-brain connectomics platform for exploring the Drosophila brain. Since 2019, scientists and experienced proofreaders have been using FlyWire to proofread AI segmentations of whole fruit fly brains. As of June 2023, more than 120,000 neurons have been proofread in FlyWire, including the entire central brain.
As shown below, the entire adult Drosophila brain reconstructed in this study contains 127,978 neurons (Figure 1a) with 53 million synapses between them. Whole-brain images of adult female Drosophila melanogaster (Fig. 1e, f) were previously obtained by serial section transfer EM and released into the public domain by Zheng et al.
 picture
picture
This article shows that the connection map obtained by reconstructing the entire Drosophila brain in this study is complete enough to be called a "connectome." The connectome is significantly improved compared to Caenorhabditis elegans (300 neurons, less than 10^4 synapses) and Drosophila first instar larvae (3,000 neurons, 5×10^5 synapses) A leap forward: the connectome not only exceeds half of the Drosophila brain in quantity, it also covers the subesophageal zone (SEZ) of the Drosophila central brain, which is very important for taste and mechanical perception. In addition, the connectome also Covers the processes that drive motor neurons from the Drosophila brain down.
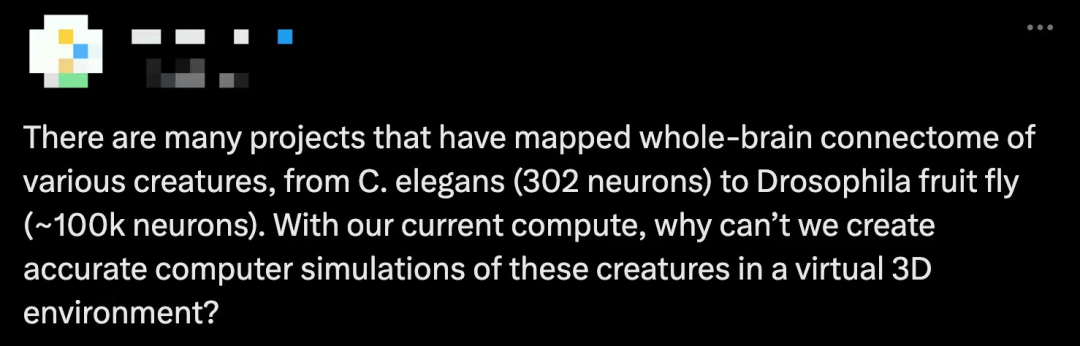
The figure below shows the categories of neurons. Figure (a) shows the classification of neurons in the Drosophila brain by "stream": intrinsic, afferent, and efferent. Each flow class is then further divided into "superclasses" based on location and function. The first publicly released compound eye was missing about 8,000 retinal cells and four tiny eyeballs in one hemisphere, parts indicated by hatched bars. (b) Using these neuronal annotations, the study created an aggregated synaptic map between superclasses in the Drosophila brain. (c) Rendering of all neurons in each superclass. (d) In addition to the ophthalmic nerve and cervical connective tissue (CV), there are 8 nerves in each hemisphere. All neurons that cross the nerve are reconstructed and explained. (e) Sensory neurons can be subdivided based on their responses to sensory modalities. In FlyWire, almost all sensory neurons have been classified by modality. (f) Rendering of all non-visual sensory neurons.
 ##Picture
##Picture
The following picture is a diagram of the fruit fly brain responsible for processing visual information:
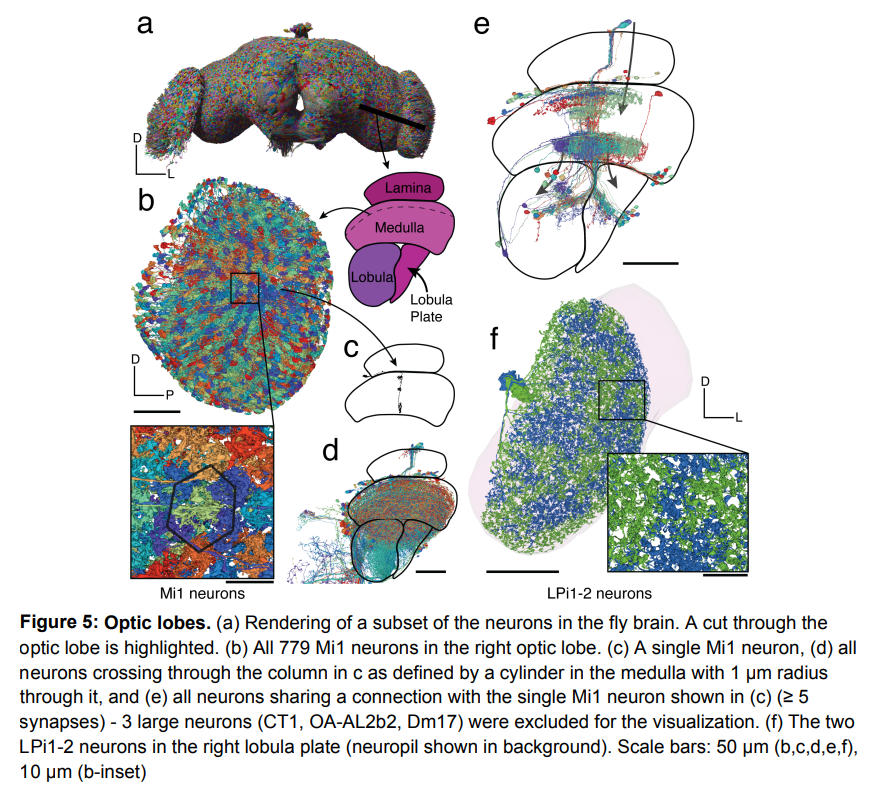 Picture
Picture
The picture below shows the flow of information through the central brain of a fruit fly:
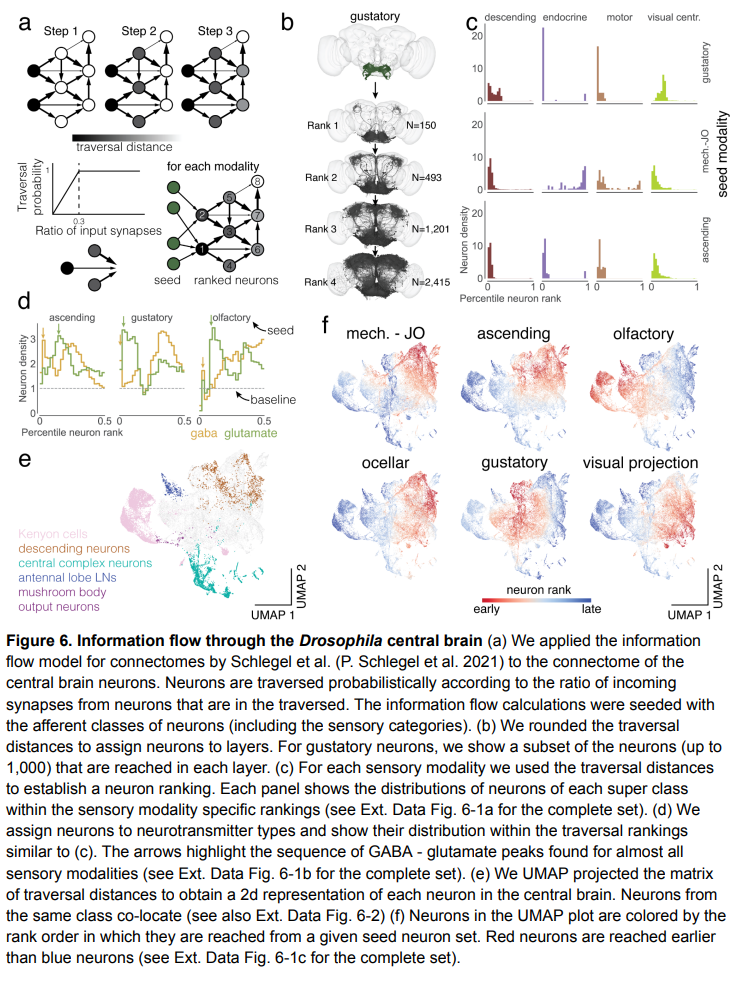 Picture
Picture
The largest ever validated collection of cell types
The FlyWire connectome is the largest and most complex connectome ever obtained. In another paper, "A consensus cell type atlas from multiple connectomes reveals principles of circuit stereotypy and variation," the team raised and answered some key questions to further explain connectomes of this scale:
1) How to know which edges are important?
2) How to simplify connectome diagrams to aid automated or manual analysis?
3) To what extent is this connectome a snapshot of a single brain or representative of the species as a whole?
 Picture
Picture
Paper link: https://www.biorxiv.org/content/10.1101/2023.06.27.546055v1. full.pdf
These questions are inextricably linked to connectome annotation and cell type identification within and across data sets.
At the most basic level, navigating this connectome would be very challenging without a comprehensive annotation system. So, the team's annotations for this paper provide an indexed and hierarchical list of human-readable parts, allowing biologists to explore the systems and neurons of interest to them.
Connectome annotation is also crucial to ensure data quality, as it inevitably reveals segmentation errors that must be corrected. Furthermore, Drosophila has a rich history of exploring the circuit basis of a wide range of innate and acquired behaviors, as well as their developmental genetic origins; realizing the full potential of the dataset will only be possible by cross-identifying cell types within the connectome with those previously identified in Characterization in published and ongoing literature.
Comparison with partial hemibrain connectomes confirms that most fly cell types are highly stereotyped; and that connections within simply defined and general heuristic connectomes are reliable and more likely to be functional sexual. However, this also revealed unexpected changes in some cell types and showed that many cell types originally reported in hemibrains cannot be reliably reidentified. This finding led to the need to develop and apply a new and powerful method to define cell types across connectomics data sets.
In this study, the researchers generated human-readable data at different levels of granularity (superclass, cell class, lineage class, etc.) for all neurons in the fly brain. Note.
The map of 4,179 cell types provided by the researchers is not the largest ever (5,620 in one half of the brain, and recent work in mouse brains provided as many as 5,000 molecular clusters) . However, it is by some means the largest validated collection of cell types ever assembled.
Cell type is a provable hypothesis about biological variability within and across animals. In C. elegans , 118 cell types inferred from the initial connectome have been unequivocally supported by subsequent analysis of connectome and molecular data. In some mammals, generating catalogs of 100 cell types is possible and has been validated with multimodal data such as in the retina or motor cortex.
However, large-scale molecular maps yield highly informative hierarchies, but no attempt has been made to precisely define terminal cell types—the finest units across individuals. The researchers tested this falsifiable cell type hypothesis for the first time on more than 5,000 cell types, confirming or correcting approximately 3,166 cell types in connectome data from 3 hemispheres.
It is worth noting that connectome data is particularly meaningful for cell type delineation: it is itself multimodal (by providing morphology and connectivity), and it can see All cells within the brain (integrity). Cell types that pass this rigorous test are very unlikely to be modified (permanently). Based on this understanding, an additional 850 cell types defined within just two hemispheres of the FlyWire dataset should also be accurate and permanent. The researchers believe that connectome data will become the gold standard for cell types. Therefore, linking molecular and connectomic cell types will be key.
People may be a little surprised or even disappointed that more than a third of half of the brain's cell types cannot yet be identified in FlyWire. The researchers reiterate that most cells can be identified, and they expect to continue to make incremental improvements on current platforms and tools through their own efforts and those of others.
Nevertheless, the current results reveal several important issues: first, many of the cell types identified using data from a single brain hemisphere now need to be modified; second, new multi-connectivity The component typing method (Figure 6) provides a powerful and efficient approach to this problem; third, examples of continuous variation in adult flies are often associated with mammalian cell types, and researchers now also have the tools and data to perform methods that were previously impossible. accuracy to handle this variation.
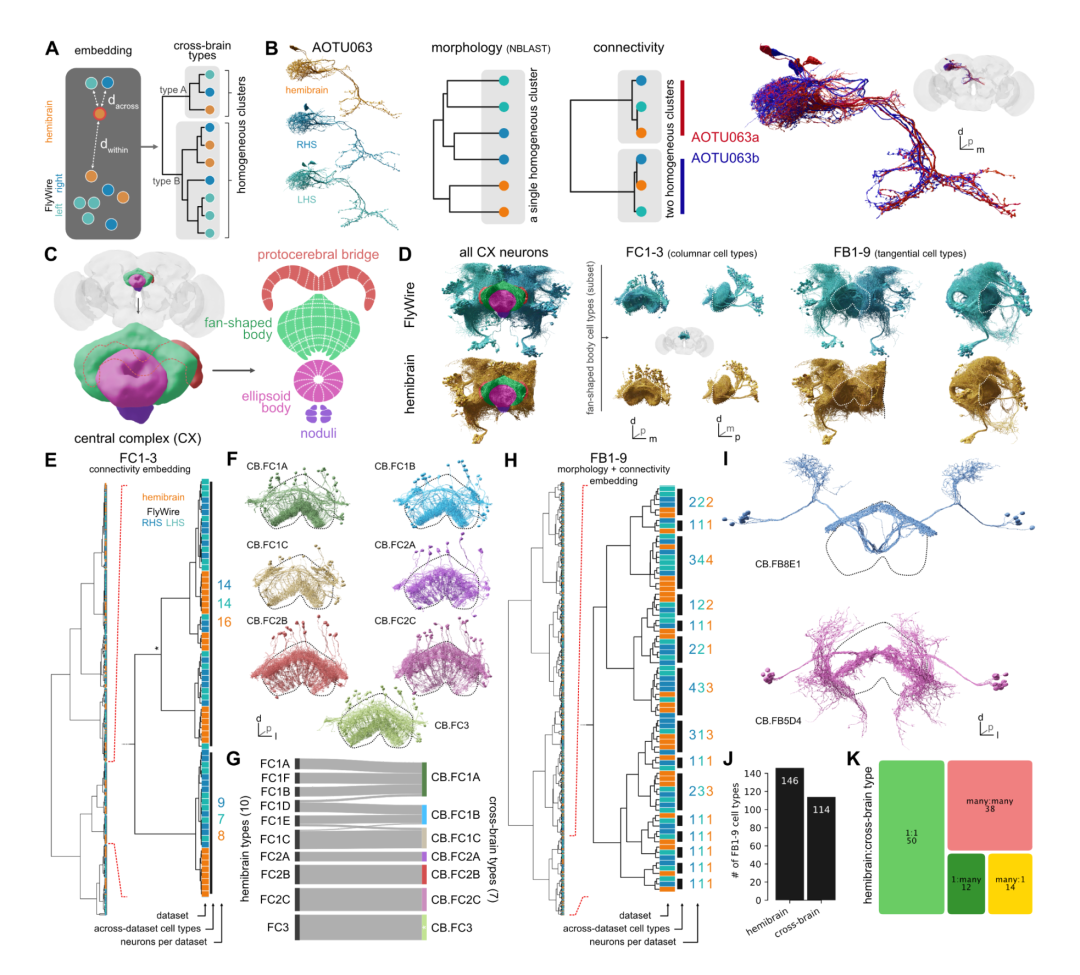 Cross-brain cell typing
Cross-brain cell typing
Overall, this work has laid some foundations for in-depth research on current and the expected normal fly connectome can also facilitate future research on sexual dimorphism, experience-dependent plasticity, whole-brain-scale development, and disease.
The above is the detailed content of With 130,000 annotated neurons and 53 million synapses, Princeton University and others released the first complete 'adult fly' brain connectome. For more information, please follow other related articles on the PHP Chinese website!

Hot AI Tools

Undresser.AI Undress
AI-powered app for creating realistic nude photos

AI Clothes Remover
Online AI tool for removing clothes from photos.

Undress AI Tool
Undress images for free

Clothoff.io
AI clothes remover

Video Face Swap
Swap faces in any video effortlessly with our completely free AI face swap tool!

Hot Article

Hot Tools

Notepad++7.3.1
Easy-to-use and free code editor

SublimeText3 Chinese version
Chinese version, very easy to use

Zend Studio 13.0.1
Powerful PHP integrated development environment

Dreamweaver CS6
Visual web development tools

SublimeText3 Mac version
God-level code editing software (SublimeText3)

Hot Topics
 1664
1664
 14
14
 1422
1422
 52
52
 1316
1316
 25
25
 1267
1267
 29
29
 1240
1240
 24
24
 2024 CSRankings National Computer Science Rankings Released! CMU dominates the list, MIT falls out of the top 5
Mar 25, 2024 pm 06:01 PM
2024 CSRankings National Computer Science Rankings Released! CMU dominates the list, MIT falls out of the top 5
Mar 25, 2024 pm 06:01 PM
The 2024CSRankings National Computer Science Major Rankings have just been released! This year, in the ranking of the best CS universities in the United States, Carnegie Mellon University (CMU) ranks among the best in the country and in the field of CS, while the University of Illinois at Urbana-Champaign (UIUC) has been ranked second for six consecutive years. Georgia Tech ranked third. Then, Stanford University, University of California at San Diego, University of Michigan, and University of Washington tied for fourth place in the world. It is worth noting that MIT's ranking fell and fell out of the top five. CSRankings is a global university ranking project in the field of computer science initiated by Professor Emery Berger of the School of Computer and Information Sciences at the University of Massachusetts Amherst. The ranking is based on objective
 The world's most powerful open source MoE model is here, with Chinese capabilities comparable to GPT-4, and the price is only nearly one percent of GPT-4-Turbo
May 07, 2024 pm 04:13 PM
The world's most powerful open source MoE model is here, with Chinese capabilities comparable to GPT-4, and the price is only nearly one percent of GPT-4-Turbo
May 07, 2024 pm 04:13 PM
Imagine an artificial intelligence model that not only has the ability to surpass traditional computing, but also achieves more efficient performance at a lower cost. This is not science fiction, DeepSeek-V2[1], the world’s most powerful open source MoE model is here. DeepSeek-V2 is a powerful mixture of experts (MoE) language model with the characteristics of economical training and efficient inference. It consists of 236B parameters, 21B of which are used to activate each marker. Compared with DeepSeek67B, DeepSeek-V2 has stronger performance, while saving 42.5% of training costs, reducing KV cache by 93.3%, and increasing the maximum generation throughput to 5.76 times. DeepSeek is a company exploring general artificial intelligence
 AI subverts mathematical research! Fields Medal winner and Chinese-American mathematician led 11 top-ranked papers | Liked by Terence Tao
Apr 09, 2024 am 11:52 AM
AI subverts mathematical research! Fields Medal winner and Chinese-American mathematician led 11 top-ranked papers | Liked by Terence Tao
Apr 09, 2024 am 11:52 AM
AI is indeed changing mathematics. Recently, Tao Zhexuan, who has been paying close attention to this issue, forwarded the latest issue of "Bulletin of the American Mathematical Society" (Bulletin of the American Mathematical Society). Focusing on the topic "Will machines change mathematics?", many mathematicians expressed their opinions. The whole process was full of sparks, hardcore and exciting. The author has a strong lineup, including Fields Medal winner Akshay Venkatesh, Chinese mathematician Zheng Lejun, NYU computer scientist Ernest Davis and many other well-known scholars in the industry. The world of AI has changed dramatically. You know, many of these articles were submitted a year ago.
 Google is ecstatic: JAX performance surpasses Pytorch and TensorFlow! It may become the fastest choice for GPU inference training
Apr 01, 2024 pm 07:46 PM
Google is ecstatic: JAX performance surpasses Pytorch and TensorFlow! It may become the fastest choice for GPU inference training
Apr 01, 2024 pm 07:46 PM
The performance of JAX, promoted by Google, has surpassed that of Pytorch and TensorFlow in recent benchmark tests, ranking first in 7 indicators. And the test was not done on the TPU with the best JAX performance. Although among developers, Pytorch is still more popular than Tensorflow. But in the future, perhaps more large models will be trained and run based on the JAX platform. Models Recently, the Keras team benchmarked three backends (TensorFlow, JAX, PyTorch) with the native PyTorch implementation and Keras2 with TensorFlow. First, they select a set of mainstream
 Hello, electric Atlas! Boston Dynamics robot comes back to life, 180-degree weird moves scare Musk
Apr 18, 2024 pm 07:58 PM
Hello, electric Atlas! Boston Dynamics robot comes back to life, 180-degree weird moves scare Musk
Apr 18, 2024 pm 07:58 PM
Boston Dynamics Atlas officially enters the era of electric robots! Yesterday, the hydraulic Atlas just "tearfully" withdrew from the stage of history. Today, Boston Dynamics announced that the electric Atlas is on the job. It seems that in the field of commercial humanoid robots, Boston Dynamics is determined to compete with Tesla. After the new video was released, it had already been viewed by more than one million people in just ten hours. The old people leave and new roles appear. This is a historical necessity. There is no doubt that this year is the explosive year of humanoid robots. Netizens commented: The advancement of robots has made this year's opening ceremony look like a human, and the degree of freedom is far greater than that of humans. But is this really not a horror movie? At the beginning of the video, Atlas is lying calmly on the ground, seemingly on his back. What follows is jaw-dropping
 KAN, which replaces MLP, has been extended to convolution by open source projects
Jun 01, 2024 pm 10:03 PM
KAN, which replaces MLP, has been extended to convolution by open source projects
Jun 01, 2024 pm 10:03 PM
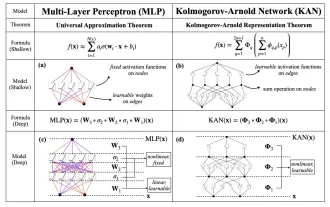
Earlier this month, researchers from MIT and other institutions proposed a very promising alternative to MLP - KAN. KAN outperforms MLP in terms of accuracy and interpretability. And it can outperform MLP running with a larger number of parameters with a very small number of parameters. For example, the authors stated that they used KAN to reproduce DeepMind's results with a smaller network and a higher degree of automation. Specifically, DeepMind's MLP has about 300,000 parameters, while KAN only has about 200 parameters. KAN has a strong mathematical foundation like MLP. MLP is based on the universal approximation theorem, while KAN is based on the Kolmogorov-Arnold representation theorem. As shown in the figure below, KAN has
 Tesla robots work in factories, Musk: The degree of freedom of hands will reach 22 this year!
May 06, 2024 pm 04:13 PM
Tesla robots work in factories, Musk: The degree of freedom of hands will reach 22 this year!
May 06, 2024 pm 04:13 PM
The latest video of Tesla's robot Optimus is released, and it can already work in the factory. At normal speed, it sorts batteries (Tesla's 4680 batteries) like this: The official also released what it looks like at 20x speed - on a small "workstation", picking and picking and picking: This time it is released One of the highlights of the video is that Optimus completes this work in the factory, completely autonomously, without human intervention throughout the process. And from the perspective of Optimus, it can also pick up and place the crooked battery, focusing on automatic error correction: Regarding Optimus's hand, NVIDIA scientist Jim Fan gave a high evaluation: Optimus's hand is the world's five-fingered robot. One of the most dexterous. Its hands are not only tactile
 DualBEV: significantly surpassing BEVFormer and BEVDet4D, open the book!
Mar 21, 2024 pm 05:21 PM
DualBEV: significantly surpassing BEVFormer and BEVDet4D, open the book!
Mar 21, 2024 pm 05:21 PM
This paper explores the problem of accurately detecting objects from different viewing angles (such as perspective and bird's-eye view) in autonomous driving, especially how to effectively transform features from perspective (PV) to bird's-eye view (BEV) space. Transformation is implemented via the Visual Transformation (VT) module. Existing methods are broadly divided into two strategies: 2D to 3D and 3D to 2D conversion. 2D-to-3D methods improve dense 2D features by predicting depth probabilities, but the inherent uncertainty of depth predictions, especially in distant regions, may introduce inaccuracies. While 3D to 2D methods usually use 3D queries to sample 2D features and learn the attention weights of the correspondence between 3D and 2D features through a Transformer, which increases the computational and deployment time.