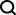Can I install HMMER software on Windows systems?

Can windows system install hmmer software
hmmer download and installation
For Mac OS/X, Linux, UNIX systems, compile and install from source code:
% wget ftp://selab.janelia.org/pub/software/hmmer3/3.0/hmmer-3.0.tar.gz % tar zxf hmmer-3.0.tar.gz % cd hmmer-3.0 % ./configure % make % make check
For Windows systems, download the binary compressed package directly, decompress it and use it.
Programs included in hmmer
phmmer: Similar to Blastp, uses a protein sequence to search the protein sequence library;
>phmmer tutorial/HBB HUMAN uniprot sprot.fa
jackhmmer: Similar to psiBlast, protein sequences iteratively search protein sequence libraries;
>jackhmmer tutorial/HBB HUMAN uniprot sprot.fa
hmmbuild: Build HMM model using multiple alignment sequences;
hmmsearch: Use HMM model to search sequence library;
hmmscan: Use sequence to search HMM library;
hmmalign: Use HMM as a clue to construct multiple alignment sequences;
>hmmalign globins4.hmm tutorial/globins45.fa
hmmconvert: Convert HMM format
hmmemit: Obtain a pattern sequence from the HMM model;
hmmfetch: Retrieve an HMM model from the HMM library by name or acceptance number;
hmmpress: Format the HMM database to facilitate hmmscan search and use;
hmmstat: Display statistical information of HMM database;
Search sequence database using HMM model
Use hmmbuild to build the HMM model, input a multiple alignment sequence file in Stockholm format or FASTA format (such as: tutorial/globins4.sto), the command is as follows:
>hmmbuild globins4.hmm tutorial/globins4.sto
globins4.hmm is the output HMM model
Use hmmsearch to search the protein sequence database. The protein sequence database is in FASTA format. The command is as follows:
>hmmsearch globins4.hmm uniprot sprot.fasta >globins4.out
globins4.out is the output result file, as follows:
*The example uses the example in the official tutorial
Search the HMM database using protein sequences
Build an HMM database. The HMM database is a file containing multiple HMM models. It can be downloaded from Pfam, SMART, and TIGRFams, or it can be built by yourself from multiple alignment sequences, such as:
>hmmbuild globins4.hmm tutorial/globins4.sto
>hmmbuild fn3.hmm tutorial/fn3.sto
>hmmbuild Pkinase.hmm tutorial/Pkinase.sto
>cat globins4.hmm fn3.hmm Pkinase.hmm >minifam
Use hmmpress to format the database, including compression and index creation. The command is as follows:
>hmmpress minifam
This step can be completed quickly, and the output content is as follows:
Working… done.
Pressed and indexed 3 HMMs (3 names and 2 accessions).
Models pressed into binary file: minifam.h3m
SSI index for binary model file: minifam.h3i
Profiles (MSV part) pressed into: minifam.h3f
Profiles (remainder) pressed into: minifam.h3p
Use hmmscan to search the HMM database, the command is as follows:
>hmmscan minifam tutorial/7LESS_DROME
How does hmmer software convert fasta format files to sto format
I also encountered this problem. I searched online for a long time and couldn't find a suitable solution, so I wrote one myself. The code is as follows
import glob # They are all things from the standard library
import os
# Put the fasta file (compared) you want to build hmm in the same folder as this program, and then run this program to run hmmbuild
os.chdir(os.path.dirname(__file__))
fs = glob.glob('*.fasta') # Get each fasta file. If your fasta file has a suffix other than .fasta, you can change it here, or directly change it to '*.fa*'
for f in fs:
hmm = os.path.splitext(f)[0] '.hmm'
stockholm = os.path.splitext(f)[0] '.sto'
with open(f, 'r') as fhandle: # This is used to read fasta files and save all fasta files to the list
fastas = ['>' tmp.replace('\n', '\r', 1).replace('\n', '').replace('\r', '\n') for tmp in tuple(filter(None, (fhandle.read().split('>'))))]
for i in range(len(fastas)):
fastas[i] = fastas[i].split('\n')
fastas[i][0] = fastas[i][0].split()[0][1:10]
tmp = []
for j in range(len(fastas[i][1]) // 80 1):
tmp.append(fastas[i][1][80 * j : 80 * j 80])
fastas[i][1] = tmp
with open(stockholm, 'w') as out: # The sto file is written here
out.write('# STOCKHOLM 1.0\n\n')
for j in range(len(fastas[0][1]) - 1):
for i in range(len(fastas)):
out.write('% -12s%s\n' % (fastas[i][0], fastas[i][1][j]))
out.write('\n')
for i in range(len(fastas)):
out.write('% -12s%s\n' % (fastas[i][0], fastas[i][1][-1]))
out.write('//')
os.system('hmmbuild --amino %s %s' % (hmm, stockholm)) # hmmbuild is running here, you can modify the parameters inside
How to teach yourself bioinformatics
1. Start with existing bioinformatics tools. Be familiar with how to use existing software, network servers, databases, etc. to serve biological research. Don’t repeat work. Don’t develop your own if you can use ready-made ones.
2. Familiar with command line operating systems, such as DOS and Linux, you can write simple shells; then you can install command line-level programs and run some regular processes. Learning how to find and install software is the most important and fundamental skill. In fact, many problems can be easily solved if you find the right software package.
3. Be familiar with a simple scripting language. I personally recommend using python. For specific reasons, please see my post. Small scripts are very useful when there are no ready-made tools, or when data format conversion is required. General applications do not need to write too much code by themselves. We must believe that other experts may have encountered the problems we usually encounter, so there are a large number of toolkits on the Internet. As for more programming languages, one can master everything, R, perl, etc. are all similar.
4. Be familiar with the knowledge of simple algorithms and data structures, so that you can understand the internal mechanisms of many programs, and then know their advantages and disadvantages, which will also be helpful for writing your own programs. If you have the energy, then study statistics, machine learning, etc. .
5. Expand, research, analyze, and develop within your own biological field.
The above is the detailed content of Can I install HMMER software on Windows systems?. For more information, please follow other related articles on the PHP Chinese website!

Hot AI Tools

Undresser.AI Undress
AI-powered app for creating realistic nude photos

AI Clothes Remover
Online AI tool for removing clothes from photos.

Undress AI Tool
Undress images for free

Clothoff.io
AI clothes remover

AI Hentai Generator
Generate AI Hentai for free.

Hot Article

Hot Tools

Notepad++7.3.1
Easy-to-use and free code editor

SublimeText3 Chinese version
Chinese version, very easy to use

Zend Studio 13.0.1
Powerful PHP integrated development environment

Dreamweaver CS6
Visual web development tools

SublimeText3 Mac version
God-level code editing software (SublimeText3)

Hot Topics
 PowerToys Is the Ultimate Work From Home Companion App
Mar 03, 2025 am 10:07 AM
PowerToys Is the Ultimate Work From Home Companion App
Mar 03, 2025 am 10:07 AM
PowerToys: Supercharge Your Windows Work-From-Home Experience Working from home presents unique productivity challenges. Distractions abound, demanding a focused workspace. PowerToys helps optimize your home office, whether it's a dedicated space or
 How to Find the Product Key for Windows 10
Mar 04, 2025 am 01:46 AM
How to Find the Product Key for Windows 10
Mar 04, 2025 am 01:46 AM
Retrieve lost Windows 10 product key: Two ways Have you lost your product key after installing genuine Windows 10? Don't worry, this article will introduce two ways to retrieve your keys so that you can reinstall the system in the future. Case 1: Windows 10 is installed but the key is lost Even if you get Windows 10 through a free upgrade or genuine installation, you can easily find the product key using the iSumsoft Product Key Finder as long as your computer works properly. Method 1: Use iSumsoft Product Key Finder Download and install: Download and install iSumsoft Product Key Finder to your computer. Find the product key: Start
 How to Install Windows 11 23H2 on Unsupported PC
Mar 03, 2025 pm 12:55 PM
How to Install Windows 11 23H2 on Unsupported PC
Mar 03, 2025 pm 12:55 PM
In the second half of 2023, Microsoft released the Windows 11 23H2 system. Many users can't wait to upgrade their computer to the latest version, but some users encountered the error message "This computer does not meet the minimum requirements..." when trying to install Windows 11 23H2 on an unsupported computer. don’t worry! This article will provide a step-by-step guide to how to install Windows 11 23H2 on an unsupported computer. Let's get started! Note: Microsoft said, "Installing Windows 11 on unsupported computers is not recommended. If you choose to install Windows 11 on hardware that does not qualify, you should risk compatibility issues." allow
 The Best Ergonomic Keyboards of 2025
Mar 03, 2025 am 10:02 AM
The Best Ergonomic Keyboards of 2025
Mar 03, 2025 am 10:02 AM
Recommended Best Ergonomic Keyboards in 2025 Ergonomic keyboards function the same as regular keyboards, but add support to reduce stress on wrists, hands and fingers. These keyboards are designed to keep your hands and wrists in a more natural position, helping to minimize muscle strain and prevent potential damage from prolonged typing. There are a wide variety of ergonomic keyboards available on the market, so be sure to consider which features are most important to you before purchasing. From layout to design, each type offers unique benefits based on your preferences and needs. Most ergonomic keyboards fall into two categories. The first category is the split keyboard, which, as the name suggests, divides the key layout into two parts for a more natural wrist posture. There are two types of split keyboards:
 Acer PD163Q Dual Portable Monitor Review: I Really Wanted to Love This
Mar 18, 2025 am 03:04 AM
Acer PD163Q Dual Portable Monitor Review: I Really Wanted to Love This
Mar 18, 2025 am 03:04 AM
The Acer PD163Q Dual Portable Monitor: A Connectivity Nightmare I had high hopes for the Acer PD163Q. The concept of dual portable displays, conveniently connecting via a single cable, was incredibly appealing. Unfortunately, this alluring idea quic
 How to Change the Font and Layout of PowerShell Window
Mar 03, 2025 pm 01:03 PM
How to Change the Font and Layout of PowerShell Window
Mar 03, 2025 pm 01:03 PM
Enhance your Windows 10 PowerShell experience with these simple customization steps! This guide shows you how to adjust PowerShell fonts and enable automatic text wrapping for improved readability and optimal window fitting. Let's get started: Step 1
 Top 3 Windows 11 Gaming Features That Outshine Windows 10
Mar 16, 2025 am 12:17 AM
Top 3 Windows 11 Gaming Features That Outshine Windows 10
Mar 16, 2025 am 12:17 AM
Upgrade to Windows 11: Enhance Your PC Gaming Experience Windows 11 offers exciting new gaming features that significantly improve your PC gaming experience. This upgrade is worth considering for any PC gamer moving from Windows 10. Auto HDR: Eleva
 How to Open File Explorer Option in Windows 10
Mar 03, 2025 pm 12:57 PM
How to Open File Explorer Option in Windows 10
Mar 03, 2025 pm 12:57 PM
Access File Explorer Options in Windows 10: Three Easy Ways This guide provides three simple methods to open File Explorer Options in Windows 10, allowing you to customize settings for file and folder views, opening items, and search functionality. M